Acromegaly and Cushing's Syndrome
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
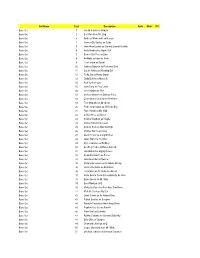
Set Name Card Description Auto Mem #'D Base Set 1 Harold Sakata As Oddjob Base Set 2 Bert Kwouk As Mr
Set Name Card Description Auto Mem #'d Base Set 1 Harold Sakata as Oddjob Base Set 2 Bert Kwouk as Mr. Ling Base Set 3 Andreas Wisniewski as Necros Base Set 4 Carmen Du Sautoy as Saida Base Set 5 John Rhys-Davies as General Leonid Pushkin Base Set 6 Andy Bradford as Agent 009 Base Set 7 Benicio Del Toro as Dario Base Set 8 Art Malik as Kamran Shah Base Set 9 Lola Larson as Bambi Base Set 10 Anthony Dawson as Professor Dent Base Set 11 Carole Ashby as Whistling Girl Base Set 12 Ricky Jay as Henry Gupta Base Set 13 Emily Bolton as Manuela Base Set 14 Rick Yune as Zao Base Set 15 John Terry as Felix Leiter Base Set 16 Joie Vejjajiva as Cha Base Set 17 Michael Madsen as Damian Falco Base Set 18 Colin Salmon as Charles Robinson Base Set 19 Teru Shimada as Mr. Osato Base Set 20 Pedro Armendariz as Ali Kerim Bey Base Set 21 Putter Smith as Mr. Kidd Base Set 22 Clifford Price as Bullion Base Set 23 Kristina Wayborn as Magda Base Set 24 Marne Maitland as Lazar Base Set 25 Andrew Scott as Max Denbigh Base Set 26 Charles Dance as Claus Base Set 27 Glenn Foster as Craig Mitchell Base Set 28 Julius Harris as Tee Hee Base Set 29 Marc Lawrence as Rodney Base Set 30 Geoffrey Holder as Baron Samedi Base Set 31 Lisa Guiraut as Gypsy Dancer Base Set 32 Alejandro Bracho as Perez Base Set 33 John Kitzmiller as Quarrel Base Set 34 Marguerite Lewars as Annabele Chung Base Set 35 Herve Villechaize as Nick Nack Base Set 36 Lois Chiles as Dr. -

ATP 2033 – Transcript
The University of Detroit mercy presents another encore presentation of a classic ask the professor radio program today's show takes us back to October 2005. Thank you so much Michael Jayson. This is Ask the Professor, the radio show where you match wits with the University of Detroit Mercy witless professors in an unrehearsed session of questions and answers here in Motown. I'd like to introduce today's panel to you and then our special guest, I should say guests in the plural. So let me start at the far end of the table from the Department of Communication Studies our media maven himself, It's Professor Jerry Curtsinger. The L.S. program. Excellent. I spent about an hour talking about that a little while ago. Trust me, who is the director? It’s Stephen Manning. Manning is. Okay, I know it's shifted. Yes, it did. It really did and I'm here because I'm not in hockey for a change. But we are so glad, but we're so glad that puck is back in our house, I couldn't stand another one of those CBC movies on Saturday night. The worst part is that Mac’s On Third I think is no more, I know. Oh, and we send out condolences first of all to Tony Bruce, and very sadly Tony's mother has passed away. So, again, our deepest sympathies and condolences to the whole Bruce family who always treated us like family. So we're sad to hear that. Truly, I know that the issue is a legalistic one, it's the famous Michigan Liquor Control Board thing that is just__ Poor Tony, so we had hopes and prayers that things work out for Tony and that someday we'll be back down at Mac’s eating some hamburgers and taping some great shows. -

Set in Scotland a Film Fan's Odyssey
Set in Scotland A Film Fan’s Odyssey visitscotland.com Cover Image: Daniel Craig as James Bond 007 in Skyfall, filmed in Glen Coe. Picture: United Archives/TopFoto This page: Eilean Donan Castle Contents 01 * >> Foreword 02-03 A Aberdeen & Aberdeenshire 04-07 B Argyll & The Isles 08-11 C Ayrshire & Arran 12-15 D Dumfries & Galloway 16-19 E Dundee & Angus 20-23 F Edinburgh & The Lothians 24-27 G Glasgow & The Clyde Valley 28-31 H The Highlands & Skye 32-35 I The Kingdom of Fife 36-39 J Orkney 40-43 K The Outer Hebrides 44-47 L Perthshire 48-51 M Scottish Borders 52-55 N Shetland 56-59 O Stirling, Loch Lomond, The Trossachs & Forth Valley 60-63 Hooray for Bollywood 64-65 Licensed to Thrill 66-67 Locations Guide 68-69 Set in Scotland Christopher Lambert in Highlander. Picture: Studiocanal 03 Foreword 03 >> In a 2015 online poll by USA Today, Scotland was voted the world’s Best Cinematic Destination. And it’s easy to see why. Films from all around the world have been shot in Scotland. Its rich array of film locations include ancient mountain ranges, mysterious stone circles, lush green glens, deep lochs, castles, stately homes, and vibrant cities complete with festivals, bustling streets and colourful night life. Little wonder the country has attracted filmmakers and cinemagoers since the movies began. This guide provides an introduction to just some of the many Scottish locations seen on the silver screen. The Inaccessible Pinnacle. Numerous Holy Grail to Stardust, The Dark Knight Scottish stars have twinkled in Hollywood’s Rises, Prometheus, Cloud Atlas, World firmament, from Sean Connery to War Z and Brave, various hidden gems Tilda Swinton and Ewan McGregor. -

Hulk © Marvel. Star Trek © Paramount. Wolf Man © Universal. Phantom © King Features
C HULK TOY, ANDY GRIFFITH’S BETTY “THELMA LOU” LYNN, TOO MUCH TV QUIZ, MR. MICROPHONE, AND MORE! AND MICROPHONE, MR. QUIZ, TV MUCH TOO LYNN, LOU” “THELMA BETTY GRIFFITH’S ANDY TOY, HULK C ELASTI ROB, MOD THE ZODY JR.”, CHANEY, LON MET I “HOW CARTOON, TREK STAR HOLLYWOOD, IN PHANTOM THE INTERVIEW, FERRIGNO LOU #1 RETROFAN 1 82658 00148 3 Hulk TM & © Marvel Characters, Inc. The Phantom © - Distributed by King Features Syndicate, Inc. Syndicate, KingFeatures by Distributed - © Phantom The Inc. Characters, TM&©Marvel Hulk Hulk © Marvel. Star Trek © Paramount. Wolf Man © Universal. Phantom © King Features. All RightsReserved. ©KingFeatures. Phantom ©Universal. Man Wolf ©Paramount. StarTrek ©Marvel. Hulk 3 CONTENTS Columns and Departments Issue #1 | Summer 2018 Special Features 2 3 Retrotorial Retro Interview Lou Ferrigno – The Incredible 9 17 Hulk Speaks! Retro Collectibles Mego’s Elastic Hulk 17 Martin Pasko’s Pesky Perspective 14 The Phantom: The Ghost Too Much TV Quiz Who Stumbles Sitcom Quotes 54 25 25 48 Andy Mangels’ Retro RetroFad Saturday Mornings Mr. Microphone Filmation’s Star Trek: The Animated Series 49 39 Retro Travel Ernest Farino’s Retro Fantasmagoria Mount Airy, NC, hometown I Met the Wolf Man! of Andy Griffith (and lived to tell about it) 59 64 54 Retro Collectibles 39 Retro Interview Andy Griffith Show Collectibles Betty Lynn – Mayberry’s Thelma Lou 72 64 Super Collector The Oddball World of Scott Shaw! Collecting Collections, by Tom Stewart Zody, the Mod Rob 80 ReJECTED RetroFan fantasy cover 49 RetroFan™ (ISSN 2576-7224), #1, Summer 2018. Published quarterly by TwoMorrows Publishing, 10407 Bedfordtown Drive, Raleigh, NC 27614. -

{FREE} Moonraker: James Bond 007 Ebook
MOONRAKER: JAMES BOND 007 PDF, EPUB, EBOOK Ian Fleming,Susan Hill | 336 pages | 02 Aug 2012 | Vintage Publishing | 9780099576020 | English | London, United Kingdom Moonraker: James Bond 007 PDF Book Universal Conquest Wiki. For Your Eyes Only was subsequently delayed and ended up following Moonraker in Bond and Goodhead disable the radar jamming cloaking device; the United States sends a platoon of Marines aboard another shuttle to intercept the now-visible space station. Mid air scrap for a parachute, a cable car sequence brilliantly realised, centrifuge chamber peril, boat chase, laser fight and much space age malarkey, the film is chocked full of crowd pleasing moments. Hugo Drax Richard Kiel While there he is attacked by Drax's henchman Chang , and they proceed to smash up most of a glass display area, before Bond throws him out the window, landing on the plaza below. Bond also meets Dr. Q: How does the movie end? Universal Conquest Wiki. Quotes [ first lines ] Captain : How are we doing, Richard? Holly Goodhead, Bond follows the trail from California to Venice, Rio de Janeiro, the Amazon rainforest , and finally into outer space to prevent a plot to wipe out the world population and to recreate humanity with a master race. Moonraker was adapted as a daily comic strip which was published in the British Daily Express newspaper and syndicated worldwide. Reviewers such as James Berardinelli praised the visual effects and stunts, [36] and film scholar James Monaco designated the film a "minor masterpiece" and declared it the best Bond film of them all. Plot Summary. Bond says 'look again, Mr. -

Goliath - the Underdog Pastor Douglas Garno September 9, 2018
Goliath - the underdog Pastor Douglas Garno September 9, 2018 Philistia Slide 2 INTRODUCTION Good morning, I'm pastor Doug Garno and I'm honored to bring today's message to you. A couple of weeks ago I was rudely interrupted by Pastor Dave during my discussion of the “Young Earth” question. So instead of a discussion about Goliath, we are going to talk about “Radiocarbon” dating or better termed Carbon 14 dating. And I have to comment on the whole “Nuclear Engineer” statement. Anyone can be one of those guys; just raise your hand and volunteered. Now for Sonar Technicians, we were the eyes and ears of the submarine. Just kidding, I had to throw that in the message this morning. Now, back to the real message. We can't read scripture like a Bookman's novel we have to investigate what is going on. We have to dig deeper to understand what God wants us to know about the story. Digging deeper also brings about how science views scripture to either prove or dis-prove some parts. Our story also shows the faith of a young boy that God will be on his side and deliver this foe for the glory of God. So I've titled the message Goliath - the underdog. Slide 3 MISS-CONCEPTION For years, I've had a miss-conception about the story of David and Goliath. I have always looked at the story where Goliath was the one who had the advantage and would easily defeat anyone who would come against him. I have to give credit where credit is due. -

Grosse Point~ ~Ws Section
Section Grosse Point~ ~ws A ----------,------------------------------ - ------------ ---~-------- --- ---- -- 30c 'er Copy 44 Page,- Three Sections 'lIbliahed oa Secoltd Cioia Molt.r -01 the GROSSE PO~TE, MICHIGAN, THURSDAY, NOVEMBER 12, 1981 $U 'er Y.or VOL. 42-NO. 46 'oat OfficI ot Delr.lt, lI"chl.on -------------------------------------- ------------------------------------------ --------- - ----------- Task force seeks school for senior housing site Heavner said senior housing is tar- obligation of that city or n()n-profit By Joanne Gouleche It's no news that enrollment in geted toward the sel1001s because, organization that leased that prop. Grosse Pointe public schools has been aeeording t() the pmposal, they are erty to develop housing upon it," the A Grosse Pointe Woods task on Ihe decline since 1971. An official Administration says it "the largest holder of land in the proposal says. count of classroom attendance in force on senior citizen housing Grosse Pointes , , . and the School Several senior groups now occupy needs has presented the Board September showed 8,195 students in Board obviously has a stewardship of space at North High School, Brownell, the system's 15 buildings - almost of Education with a proposal to ,viII study request this commun ity's most unavailable Parcells and Ferry schools. turn unused school building sites 5,000 fewer than in 1970. School of- resource." ficials have not seen those figures The Grosse Pointe Woods based Sen. into f~ture housing for Grosse since the 1950s, and it has prompted The housing proposal specifically iors Onward for Change (SOC) Food Pointe's elderly. asks that when a school building is a study of enrollment decline an\! .over the past 10 years (more than an and Friendship pwgram works OIit future use of school buildings. -

Movie Trivia
------------------------------------------------------------------------------- MOVIE TRIVIA Frequently Asked Questions Copyright (C) 1992-5 Murray Chapman ------------------------------------------------------------------------------- Compiled by Murray Chapman ([email protected]), from sources too numerous too mention. Thank-you one and all. INTRODUCTION ------------ This is a list of interesting trivia, ``did you notice’’-type things for movies. This list is part of The Internet Movie Database. See the notes at the end for more information. This, and MANY other FAQs are available for anonymous FTP wherever news.answers is archived, for example: rtfm.mit.edu:/pub/usenet/news.answers/movies/trivia-faq The followup field is set to rec.arts.movies. Additions and suggestions welcome: if you can confirm any rumors, or dispute any ``facts’’, then please do so! PLEASE read the notes at end before you submit anything. This is becoming increasingly important. Thanks! DISCLAIMER ---------- The data contained in this file has been supplied by numerous sources, many of which are anonymous and second- or third-hand. By its very nature, the data contained herein is particularly susceptible to innuendo and rumor. While I have exercised considerable editorial control by: a) attempting to eliminate scandal, sensationalism, and/or slander, b) seeking confirmatin of rumours, and c) expressing a willingness to debate the validity of included data, I will not (and could not possibly be expected to) accept responsibility or liability for any views/claims/rumours/errors that appears herein. The views expressed in this file do not necessarily agree with my own. I have attempted to present information in a professional and non-sensationalist manner, but as far as the information itself goes, I am obviously at the mercy of those who supply the data. -

Film Detective Releases Special Edition of Eegah Plus New
The future delivered now. Allied Vaughn. Powering today's leading entertainment supply chain. Volume 9 Issue 20 This week we celebrate the addition of Screen Media Ventures and 3rd Fathom studios to the exclusive catalog of Allied Vaughn Entertainment offerings for our Retailer customers. You'll find their content worthy of your Holiday selling efforts. We also salute our friends at The Film Detective for bringing "EEGAH" to Blu-ray in a limited release, beautifully mastered with bonus features sure to please the most selective film collector. The late Richard Kiel, better known as Jaws in the James Bond films, was a favorite at conventions around the world. I had the meet Richard on several occasions and he was a warm, charming man. From our major studios, Warner, FOX, MGM, Universal, CBS, Sony, Paramount and our Independents, you'll find our 100% ready to ship distribution model the fastest growing sales channel option for physical retail! Richard Skillman Vice President Allied Vaughn Entertainment [email protected] Allied Vaughn Entertainment Main Page Allied Vaughn Entertainment Studio Catalog Allied Vaughn Entertainment Archives Sony Expands Blu-ray Classics Catalog 11/19/2019 043396563575 The Whole Nine Yards [Blu-ray] 2001 Fresh out of prison, seasoned criminal Michael Zane wastes no time setting up his next big heist by joining up with a new gang led by his old friend and partner-in-crime, Thomas Murphy. Along with Hanson, Franklin and Gus, the boys plan a daring raid on the Riviera Hotel Casino in Las Vegas during International Elvis Week. Decked out in high collars, glittering belted jumpsuits and sideburns for miles, the gang hits the strip in search of their jackpot. -

Interview with Martin Grace (Roger Moore Stunt Double) - 2009/2010
INTERVIEW WITH MARTIN GRACE (ROGER MOORE STUNT DOUBLE) - 2009/2010 Interview by Anders Frejdh, Founder of From Sweden with Love, and Marie-France Vienne, webmaster for Roger Moore’s official website. First of all, could you tell us a little bit about yourself? I was born in Kilkenny, Ireland on September 12, 1942. I attended National School at a little place called Lisdowney. The village master in the town thought his little school were the best. He was a sincere man and stern to view. That was from the age of 6 to 14. Then, I attended the college in Kilkenny City. It was during this time that I experienced the excitement of watching my first action movies by a travelling film show in a tent. It was sheer magic. I went to London in the early 1960’s and attended Mountview Academy of Theatre Arts. I took a job with Butlins Holiday Camp in 1963, organising sport and playing in their theatre to gain experience. I won a Charlton Heston talent contest in 1974 that took me to Hollywood for the first time. I was already preparing my skills with boxing, wrestling, fencing, swimming, trampoline and gym work out, special motorcar and motorcycling driving skills, parachuting and various other sports in view of becoming an action stunt man in films. I attended stunt classes to learn the basics and joined a stunt agency for films and commercials. It was for commercials that I got my first stunt jobs. The white knight in Supersoft hairspray and the action man on Cadburys milk tray chocolates stunts. -

No. 07-1712 ___WRS INC, D/B/A WRS M
Case: 07-1712 Document: 00311974493 Page: 1 Date Filed: 07/18/2008 NOT PRECEDENTIAL UNITED STATES COURT OF APPEALS FOR THE THIRD CIRCUIT ____________ No. 07-1712 ____________ WRS INC, d/b/a WRS MOTION PICTURE LABORATORIES, a corporation v. PLAZA ENTERTAINMENT, INC, a corporation; ERIC PARKINSON, an individual; CHARLES VON BERNUTH; JOHN HERKLOTZ, an individual John Herklotz, Appellant ____________ On Appeal from the United States District Court for the Western District of Pennsylvania (No. 00-cv-2041) District Judge: Hon. Arthur J. Schwab Submitted Under Third Circuit LAR 34.1(a) June 6, 2008 Before: AMBRO, CHAGARES, and COWEN, Circuit Judges. ____________ (Filed: July 18, 2008) OPINION OF THE COURT Case: 07-1712 Document: 00311974493 Page: 2 Date Filed: 07/18/2008 CHAGARES, Circuit Judge. John Herklotz appeals two summary judgment decisions of the District Court. The first found Herklotz liable under a surety agreement, while the second fixed the amount of damages and attorneys’ fees due Herklotz’s opponent. We will affirm the District Court’s decision on liability. We will remand the damages decision for further proceedings in light of the District Court’s indication that it will, upon remand, grant Herklotz’s motion for relief under Federal Rule of Civil Procedure 60(b)(6) regarding that decision. I. In 1996, Eric Parkinson formed Plaza Entertainment (Plaza), a company that obtained the rights to movie titles and then duplicated, distributed, and otherwise exploited those titles. Herklotz and Charles von Bernuth joined Parkinson as the three shareholders in Plaza, and Herklotz served as Plaza’s C.E.O. -

The 50 Greatest Heroes and the 50 Greatest Villains of All Time 400 Nominated Characters
The 50 greatest heroes and the 50 greatest villains of all time 400 Nominated Characters 1 BUDDY ACKERMAN in SWIMMING WITH SHARKS (Trimark, 1994) ACTOR Kevin Spacey DIRECTOR George Huang PRODUCERS Steve Alexander, Joanne Moore SCREENWRITERGeorge Huang COSTUMES Kirsten Everberg MAKE-UP Sarah Gaye Deal HAIR Sarah Gaye Deal 2 SHEIK AHMED in THE SHEIK (Paramount, 1921) ACTOR Rudolph Valentino DIRECTOR George Melford PRODUCER Jesse L. Lasky SCREENWRITERMonte M. Katterjohn ALSO appears in: THE SON OF THE SHEIK (United Artists, 1926) 3 THE ALIEN in ALIEN (20th Century Fox, 1979) ACTOR Bolaji Badejo DIRECTOR Ridley Scott PRODUCERS Gordon Carroll, David Giler, Walter Hill SCREENWRITERDan O’Bannon ALIEN DESIGN H. R. Giger 4 JAMES ALLEN in I AM A FUGITIVE FROM A CHAIN GANG (Warner Bros., 1932) ACTOR Paul Muni DIRECTOR Mervyn LeRoy PRODUCER Hal B. Wallis SCREENWRITERS Howard J. Green, Brown Holmes AFI is a trademark of the American Film Institute. Copyright 2005 American Film Institute. All Rights Reserved. 5 CRYSTAL ALLEN in THE WOMEN (MGM, 1939) ACTOR Joan Crawford DIRECTOR George Cukor PRODUCER Hunt Stromberg SCREENWRITERS Anita Loos, Jane Murfin COSTUMES Adrian MAKE-UP Sydney Guilaroff 6 GREGORY ANTON in GASLIGHT (MGM, 1944) ACTOR Charles Boyer DIRECTOR George Cukor PRODUCER Arthur Hornblow, Jr. SCREENWRITERS John Van Druten, Walter Reisch, John L. Balderston COSTUMES Irene MAKE-UP Jack Dawn 7 BRUNO ANTONY in STRANGERS ON A TRAIN (Warner Bros., 1951) ACTOR Robert Walker DIRECTOR Alfred Hitchcock PRODUCER Alfred Hitchcock SCREENWRITERRaymond Chandler, Czenzi Ormonde COSTUMES Leah Rhodes MAKE-UP Gordon Bau 8 DAVID ARMSTRONG in WINGS (Paramount, 1927) ACTOR Richard Arlen DIRECTOR William A.