In Situ Solvent Assisted and Solvent-Based Recovery: Workshop 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cenovus Reports Second-Quarter 2020 Results Company Captures Value by Leveraging Flexibility of Its Operations Calgary, Alberta (July 23, 2020) – Cenovus Energy Inc
Cenovus reports second-quarter 2020 results Company captures value by leveraging flexibility of its operations Calgary, Alberta (July 23, 2020) – Cenovus Energy Inc. (TSX: CVE) (NYSE: CVE) remained focused on financial resilience in the second quarter of 2020 and used the flexibility of its assets and marketing strategy to adapt quickly to the changing external environment. This positioned the company to weather the sharp decline in benchmark crude oil prices in April by reducing volumes at its oil sands operations and storing the mobilized oil in its reservoirs for production in an improved price environment. While Cenovus’s financial results were impacted by the weak prices early in the quarter, the company captured value by quickly ramping up production when Western Canadian Select (WCS) prices increased almost tenfold from April to an average of C$46.03 per barrel (bbl) in June. As a result of this decision, Cenovus reached record volumes at its Christina Lake oil sands project in June and achieved free funds flow for the month of more than $290 million. “We view the second quarter as a period of transition, with April as the low point of the downturn and the first signs of recovery taking hold in May and June,” said Alex Pourbaix, Cenovus President & Chief Executive Officer. “That said, we expect the commodity price environment to remain volatile for some time. We believe the flexibility of our assets and our low cost structure position us to withstand a continued period of low prices if necessary. And we’re ready to play a significant -

Cenovus Energy Inc. (CVE) – Quality and Growth for the Patient Investor
Portfolio Advisory Group Cenovus Energy Inc. (CVE) – Quality and Growth For The Patient Investor Cenovus was created through the split of Calgary-based Primer on the Oil Sands and energy company EnCana into two separate organizations in late 2009. As a result of this split, many Canadian Steam-Assisted-Gravity-Drainage investors found themselves holding two very different (SAGD) investments: a pure-play natural gas company (EnCana) Including the oil sands, Canada’s oil reserves are the and an integrated oil & gas company (Cenovus). This second largest in the world. Oil sands are composed document aims to provide some insight into the nature primarily of sand, clay, bitumen and water. Bitumen and long-term potential of Cenovus. is the product of oil sands production – a thick oil Cenovus owns oil sands projects that have a tremendous embedded in sand. growth profile over the next decade and are widely Recovery of bitumen is typically achieved by one of viewed as some of the highest-quality assets in the two methods: mining in open pits or drilling. Bitumen industry. However, since inception, the shares of extraction using drilling is referred to as in situ recovery, Cenovus have largely traded within a range. One reason and is generally used for reservoirs that are too deep for is that the company has made a trade-off between near- surface mining techniques to work economically. It is term and future production by drawing cash flows from estimated that approximately 80% of the total bitumen its natural gas business to fund growth in its oil sands recoverable in Alberta can only be produced with in business. -

Cenovus Completes Acquisition of Assets in Western Canada from Conocophillips
Cenovus completes acquisition of assets in Western Canada from ConocoPhillips Calgary, Alberta (May 17, 2017) – Cenovus Energy Inc. (TSX: CVE) (NYSE: CVE) has closed its previously announced purchase of assets in Western Canada from ConocoPhillips after receiving all necessary regulatory approvals for the transaction. The acquired assets include ConocoPhillips’ 50% interest in the FCCL Partnership, the oil sands venture which was jointly owned with and operated by Cenovus, as well as the majority of ConocoPhillips’ Deep Basin conventional assets in Alberta and British Columbia. “With the completion of this transformational deal, we now have full control of our best-in- class oil sands projects and an exciting new growth platform in the Deep Basin that provides us with significant short-cycle development opportunities to complement our long-term oil sands growth portfolio,” said Brian Ferguson, Cenovus President & Chief Executive Officer. “As a result of this transaction, we’ve now doubled our production and reserves base.” In the coming months, Cenovus will remain firmly focused on: • Continuing to safely and reliably operate all of its assets • Efficiently integrating the Deep Basin assets and staff into the company • Deleveraging its balance sheet, including using the proceeds of planned divestitures, such as the sale of the company’s Pelican Lake and Suffield assets, which are currently being marketed. Cenovus intends to provide an update on its investment plans for its consolidated oil sands business and newly acquired Deep Basin -

Canadian Crude Oil at Record Discount
Canadian crude oil at record discount Hilliard MacBeth Hilliard’s Weekend Notebook – Friday October 19, 2018 Richardson GMP World oil prices are at cycle highs recently with the benchmark Brent at US$80 and 10180 101 Street, Suite 3360 the North American West Texas Intermediate (WTI) at US$70. Edmonton, AB T5J 3S4 Tel. 1.780.409.7735 But in Canada, the Western Canada Select (WCS) price is below US$20. Fax 780.409.7777 www.TheMacBethGroup.com This gap of US$50 to WTI is shocking. Hilliard MacBeth Will the price for a key Canadian energy export recover soon enough to save the Director, Wealth Management industry? Portfolio Manager Tel. 780.409.7740 Source: Bloomberg Rising production from oil sands along with a lack of pipeline capacity to move that product to the US market gets the blame for this extremely low price. On October 12, the price for WCS fell to US$15.97, as of yesterday the price has returned to US$19.75. Source: OilPrice.com One solution that should have been in place by now is the Keystone XL pipeline that was approved years ago but keeps hitting new roadblocks. This pipeline is the 4th phase of an existing pipeline system that moves crude to the US refineries that are well-suited to handle Alberta’s heavy crude. The new XL phase would handle an additional 830,000 barrels per day but won’t be on line until 2021, if it goes ahead. Nebraska’s highest court has yet to rule on another legal challenge to the route. -

. . Expanding the Mission . . . Facilitating the Future PETROLEUM TECHNOLOGY ALLIANCE CANADA
PETROLEUM TECHNOLOGY ALLIANCE CANADA ANNUAL REPOR T 2007 . expanding the mission . facilitating the future PETROLEUM TECHNOLOGY ALLIANCE CANADA Mission PTAC facilitates innovation, collaborative research and technology development, demonstration and deployment for a responsible Canadian hydrocarbon energy industry. Technical Areas Enhanced Environmental Management Emission Reduction / Eco-Efficiency Energy Efficiency Resource Access Air Ecological Soil and Groundwater Water Improved Oil and Gas Recovery Conventional Oil and Gas Recovery CO2 Enhanced Hydrocarbon Recovery Coalbed Methane/Unconventional Gas Oil Sands Heavy Oil Reservoir Engineering Geosciences Cost Reductions/Operations Alternative Energy Production Engineering Facility Design Drilling and Well Completion Inactive Wells Instrumentation / Measurement E-Business Fundamental Research Health and Safety Innovation R&D Funding Security Telecommunications Upgrading, Refining, Petrochemical Technologies, and Transportation Hydrocarbon Upgrading Refining Petrochemicals Hydrogen Gasification Pipelines Integration Transportation ANNUAL REPOR T 2 0 0 7 . expanding oppor tunities . 2007 Key Accomplishments Message from the Board of Directors Established a new vision: to help Canada The hydrocarbon energy sector in Canada has experienced significant become a global hydrocarbon energy changes since PTAC was first established, and in 2007 our commitment to our technology leader. This vision is supported members led us to examine our organization and the role we play in the indus- by a broadened -

Annual Information Form 2014 March 9, 2015
ANNUAL INFORMATION FORM 2014 MARCH 9, 2015 TABLE OF CONTENTS Page SELECTED TERMS ..................................................................................................................................................... 1 ABBREVIATIONS ....................................................................................................................................................... 4 CONVERSIONS ........................................................................................................................................................... 4 CONVENTIONS ........................................................................................................................................................... 5 SPECIAL NOTES TO READER .................................................................................................................................. 5 BAYTEX ENERGY CORP. ......................................................................................................................................... 9 GENERAL DEVELOPMENT OF OUR BUSINESS ................................................................................................. 10 RISK FACTORS ......................................................................................................................................................... 11 DESCRIPTION OF OUR BUSINESS AND OPERATIONS ..................................................................................... 11 DIRECTORS AND OFFICERS ................................................................................................................................. -

Foreign Investment in the Oil Sands and British Columbia Shale Gas
Canadian Energy Research Institute Foreign Investment in the Oil Sands and British Columbia Shale Gas Jon Rozhon March 2012 Relevant • Independent • Objective Foreign Investment in the Oil Sands and British Columbia Shale Gas 1 Foreign Investment in the Oil Sands There has been a steady flow of foreign investment into the oil sands industry over the past decade in terms of merger and acquisition (M&A) activity. Out of a total CDN$61.5 billion in M&A’s, approximately half – or CDN$30.3 billion – involved foreign companies taking an ownership stake. These funds were invested in in situ projects, integrated projects, and land leases. As indicated in Figure 1, US and Chinese companies made the most concerted efforts to increase their profile in the oil sands, investing 2/3 of all foreign capital. The US and China both invested in a total of seven different projects. The French company, Total SA, has also spread its capital around several projects (four in total) while Royal Dutch Shell (UK), Statoil (Norway), and PTT (Thailand) each opted to take large positions in one project each. Table 1 provides a list of all foreign investments in the oil sands since 2004. Figure 1: Total Oil Sands Foreign Investment since 2003, Country of Origin Korea 1% Thailand Norway 6% UK 7% 2% US France 33% 18% China 33% Source: Canoils. Foreign Investment in the Oil Sands and British Columbia Shale Gas 2 Table 1: Oil Sands Foreign Investment Deals Year Country Acquirer Brief Description Total Acquisition Cost (000) 2012 China PetroChina 40% interest in MacKay River 680,000 project from AOSC 2011 China China National Offshore Acquisition of OPTI Canada 1,906,461 Oil Corporation 2010 France Total SA Alliance with Suncor. -

Climate and Energy Benchmark in Oil and Gas
Climate and Energy Benchmark in Oil and Gas Total score ACT rating Ranking out of 100 performance, narrative and trend 1 Neste 57.4 / 100 8.1 / 20 B 2 Engie 56.9 / 100 7.9 / 20 B 3 Naturgy Energy 44.8 / 100 6.8 / 20 C 4 Eni 43.6 / 100 7.3 / 20 C 5 bp 42.9 / 100 6.0 / 20 C 6 Total 40.7 / 100 6.1 / 20 C 7 Repsol 38.1 / 100 5.0 / 20 C 8 Equinor 37.9 / 100 4.9 / 20 C 9 Galp Energia 36.4 / 100 4.3 / 20 C 10 Royal Dutch Shell 34.3 / 100 3.4 / 20 C 11 ENEOS Holdings 32.4 / 100 2.6 / 20 C 12 Origin Energy 29.3 / 100 7.3 / 20 D 13 Marathon Petroleum Corporation 24.8 / 100 4.4 / 20 D 14 BHP Group 22.1 / 100 4.3 / 20 D 15 Hellenic Petroleum 20.7 / 100 3.7 / 20 D 15 OMV 20.7 / 100 3.7 / 20 D Total score ACT rating Ranking out of 100 performance, narrative and trend 17 MOL Magyar Olajes Gazipari Nyrt 20.2 / 100 2.5 / 20 D 18 Ampol Limited 18.8 / 100 0.9 / 20 D 19 SK Innovation 18.6 / 100 2.8 / 20 D 19 YPF 18.6 / 100 2.8 / 20 D 21 Compania Espanola de Petroleos SAU (CEPSA) 17.9 / 100 2.5 / 20 D 22 CPC Corporation, Taiwan 17.6 / 100 2.4 / 20 D 23 Ecopetrol 17.4 / 100 2.3 / 20 D 24 Formosa Petrochemical Corp 17.1 / 100 2.2 / 20 D 24 Cosmo Energy Holdings 17.1 / 100 2.2 / 20 D 26 California Resources Corporation 16.9 / 100 2.1 / 20 D 26 Polski Koncern Naftowy Orlen (PKN Orlen) 16.9 / 100 2.1 / 20 D 28 Reliance Industries 16.7 / 100 1.0 / 20 D 29 Bharat Petroleum Corporation 16.0 / 100 1.7 / 20 D 30 Santos 15.7 / 100 1.6 / 20 D 30 Inpex 15.7 / 100 1.6 / 20 D 32 Saras 15.2 / 100 1.4 / 20 D 33 Qatar Petroleum 14.5 / 100 1.1 / 20 D 34 Varo Energy 12.4 / 100 -

Big Oil's Oily Grasp
Big Oil’s Oily Grasp The making of Canada as a Petro-State and how oil money is corrupting Canadian politics Daniel Cayley-Daoust and Richard Girard Polaris Institute December 2012 The Polaris Institute is a public interest research organization based in Canada. Since 1997 Polaris has been dedicated to developing tools and strategies to take action on major public policy issues, including the corporate power that lies behind public policy making, on issues of energy security, water rights, climate change, green economy and global trade. Polaris Institute 180 Metcalfe Street, Suite 500 Ottawa, ON K2P 1P5 Phone: 613-237-1717 Fax: 613-237-3359 Email: [email protected] www.polarisinstitute.org Cover image by Malkolm Boothroyd Table of Contents Introduction 1 1. Corporations and Industry Associations 3 2. Lobby Firms and Consultant Lobbyists 7 3. Transparency 9 4. Conclusion 11 Appendices Appendix A, Companies ranked by Revenue 13 Appendix B, Companies ranked by # of Communications 15 Appendix C, Industry Associations ranked by # of Communications 16 Appendix D, Consultant lobby firms and companies represented 17 Appendix E, List of individual petroleum industry consultant Lobbyists 18 Appendix F, Recurring topics from communications reports 21 References 22 ii Glossary of Acronyms AANDC Aboriginal Affairs and Northern Development Canada CAN Climate Action Network CAPP Canadian Association of Petroleum Producers CEAA Canadian Environmental Assessment Act CEPA Canadian Energy Pipelines Association CGA Canadian Gas Association DPOH -

Impacts and Mitigations of in Situ Bitumen Production from Alberta Oil Sands
Impacts and Mitigations of In Situ Bitumen Production from Alberta Oil Sands Neil Edmunds, P.Eng. V.P. Enhanced Oil Recovery Laricina Energy Ltd. Calgary, Alberta Submission to the XXIst World Energy Congress Montréal 2010 - 1 - Introduction: In Situ is the Future of Oil Sands The currently recognized recoverable resource in Alberta’s oil sands is 174 billion barrels, second largest in the world. Of this, about 150 billion bbls, or 85%, is too deep to mine and must be recovered by in situ methods, i.e. from drill holes. This estimate does not include any contributions from the Grosmont carbonate platform, or other reservoirs that are now at the early stages of development. Considering these additions, together with foreseeable technological advances, the ultimate resource potential is probably some 50% higher, perhaps 315 billion bbls. Commercial in situ bitumen recovery was made possible in the 1980's and '90s by the development in Alberta, of the Steam Assisted Gravity Drainage (SAGD) process. SAGD employs surface facilities very similar to steamflooding technology developed in California in the ’50’s and 60’s, but differs significantly in terms of the well count, geometry and reservoir flow. Conventional steamflooding employs vertical wells and is based on the idea of pushing the oil from one well to another. SAGD uses closely spaced pairs of horizontal wells, and effectively creates a melt cavity in the reservoir, from which mobilized bitumen can be collected at the bottom well. Figure 1. Schematic of a SAGD Well Pair (courtesy Cenovus) Economically and environmentally, SAGD is a major advance compared to California-style steam processes: it uses about 30% less steam (hence water and emissions) for the same oil recovery; it recovers more of the oil in place; and its surface impact is modest. -
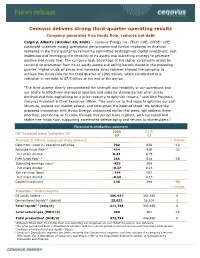
Cenovus Delivers Strong Third-Quarter Operating Results Company Generates Free Funds Flow, Reduces Net Debt Calgary, Alberta (October 29, 2020) – Cenovus Energy Inc
Cenovus delivers strong third-quarter operating results Company generates free funds flow, reduces net debt Calgary, Alberta (October 29, 2020) – Cenovus Energy Inc. (TSX: CVE) (NYSE: CVE) continued to deliver strong operational performance and further improved its financial resilience in the third quarter by remaining committed to disciplined capital investment, cost leadership and leveraging the flexibility of its assets and marketing strategy to generate positive free funds flow. The company took advantage of the higher commodity prices by ramping up production from its oil sands assets and selling barrels stored in the preceding quarter. Higher crude oil prices and increased sales volumes allowed the company to achieve free funds flow for the third quarter of $266 million, which contributed to a reduction in net debt to $7.5 billion at the end of the period. “The third quarter clearly demonstrated the strength and reliability of our operations and our ability to effectively manage production and sales by storing barrels when prices declined and then capitalizing on a price recovery to optimize returns,” said Alex Pourbaix, Cenovus President & Chief Executive Officer. “We continue to find ways to optimize our cost structure, expand our market access, and strengthen the balance sheet. We believe the proposed transaction with Husky Energy, announced earlier this week, will address these priorities, positioning us to come through this period more resilient, with increased and stable free funds flow, supporting accelerated deleveraging -
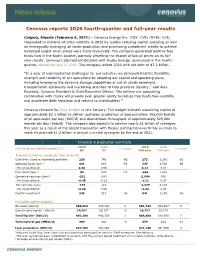
Cenovus Reports 2020 Fourth-Quarter and Full-Year Results
Cenovus reports 2020 fourth-quarter and full-year results Calgary, Alberta (February 9, 2021) – Cenovus Energy Inc. (TSX: CVE) (NYSE: CVE) responded to extreme oil price volatility in 2020 by quickly reducing capital spending as well as strategically managing oil sands production and purchasing curtailment credits to achieve increased output when prices were more favourable. The company generated positive free funds flow in the fourth quarter, partially offsetting the impact of low oil prices on its full- year results. Cenovus’s planned combination with Husky Energy, announced in the fourth quarter, closed January 1, 2021. The company exited 2020 with net debt of $7.2 billion. “In a year of unprecedented challenges for our industry, we demonstrated the flexibility, strength and reliability of our operations by adapting our capital and operating plans, including leveraging the dynamic storage capabilities of our oil sands reservoirs, transportation optionality and marketing activities to help preserve liquidity,” said Alex Pourbaix, Cenovus President & Chief Executive Officer. “We believe our compelling combination with Husky will provide even greater ability to reduce free funds flow volatility and accelerate debt reduction and returns to shareholders.” Cenovus released its 2021 budget in late January. The budget includes sustaining capital of approximately $2.1 billion to deliver upstream production of approximately 755,000 barrels of oil equivalent per day (BOE/d) and downstream throughput of approximately 525,000 barrels per day (bbls/d). The company also expects to achieve nearly $1 billion of synergies this year as a result of the recent transaction with Husky, putting Cenovus firmly on track to reach its planned $1.2 billion in annual run-rate synergies by the end of 2021.