Kororā Monitoring Protocols Version 2 | April 2021
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bruxner Park Flora Reserve Working Plan
Bruxner Park Flora Reserve Working Plan Working Plan for Bruxner Park Flora Reserve No 3 Upper North East Forest Agreement Region North East Region Contents Page 1. DETAILS OF THE RESERVE 2 1.1 Introduction 2 1.2 Location 2 1.3 Key Attributes of the Reserve 2 1.4 General Description 2 1.5 History 6 1.6 Current Usage 8 2. SYSTEM OF MANAGEMENT 9 2.1 Objectives of Management 9 2.2 Management Strategies 9 2.3 Management Responsibility 11 2.4 Monitoring, Reporting and Review 11 3. LIST OF APPENDICES 11 Appendix 1 Map 1 Locality Appendix 1 Map 2 Cadastral Boundaries, Forest Types and Streams Appendix 1 Map 3 Vegetation Growth Stages Appendix 1 Map 4 Existing Occupation Permits and Recreation Facilities Appendix 2 Flora Species known to occur in the Reserve Appendix 3 Fauna records within the Reserve Y:\Tourism and Partnerships\Recreation Areas\Orara East SF\Bruxner Flora Reserve\FlRWP_Bruxner.docx 1 Bruxner Park Flora Reserve Working Plan 1. Details of the Reserve 1.1 Introduction This plan has been prepared as a supplementary plan under the Nature Conservation Strategy of the Upper North East Ecologically Sustainable Forest Management (ESFM) Plan. It is prepared in accordance with the terms of section 25A (5) of the Forestry Act 1916 with the objective to provide for the future management of that part of Orara East State Forest No 536 set aside as Bruxner Park Flora Reserve No 3. The plan was approved by the Minister for Forests on 16.5.2011 and will be reviewed in 2021. -

Olm003) District 02 ------Lease Name--- Field Name Organization Lease Name Number ------L- Ranch Lolita (Marginulina Zone) Miner, R
AUG 01, 2020 OIL LEASE NAME INDEX LISTING PAGE 189 (OLM003) DISTRICT 02 ------------------------------------------------------------------------------------------------------------------------------------ ---LEASE NAME--- FIELD NAME ORGANIZATION LEASE NAME NUMBER ------------------------------------------------------------------------------------------------------------------------------------ -L- RANCH LOLITA (MARGINULINA ZONE) MINER, R. C. OIL, INC. 02971 -L- RANCH LOLITA (WARD ZONE) MINER, R. C. OIL, INC. 06484 -L- RANCH CO. -A- LOLITA (BENNVIEW ZONE) MCGOWAN WORKING PARTNERS, INC. 04132 -L- RANCH CO. -A- LOLITA (MARGINULINA ZONE) MCGOWAN WORKING PARTNERS, INC. 00874 -L- RANCH CO. -A- LOLITA (MINER SAND) MCGOWAN WORKING PARTNERS, INC. 05840 -L- RANCH CO. -A- LOLITA (MOPAC SAND) MCGOWAN WORKING PARTNERS, INC. 02229 -L- RANCH CO. -A- LOLITA (TONEY ZONE) MCGOWAN WORKING PARTNERS, INC. 00903 -L- RANCH CO. -A- LOLITA (41-A) MCGOWAN WORKING PARTNERS, INC. 04269 -L- RANCH CO. -B- LOLITA (MARGINULINA ZONE) MCGOWAN WORKING PARTNERS, INC. 00875 'BULL REDFISH' UNIT PANTHER REEF (FRIO CONS.) MAGNUM OPERATING, LLC 11235 A J WILLOUGHBY LOLITA MCGOWAN WORKING PARTNERS, INC. 10358 A MUELLER A-KOEHLER SW SUGARKANE (AUSTIN CHALK) BURLINGTON RESOURCES O & G CO LP 11814 A MUELLER A-KOEHLER SW A EAGLEVILLE (EAGLE FORD-2) BURLINGTON RESOURCES O & G CO LP 11835 A MUELLER A-KOEHLER SW B EAGLEVILLE (EAGLE FORD-2) BURLINGTON RESOURCES O & G CO LP 11807 A MUELLER A-KOEHLER SW C EAGLEVILLE (EAGLE FORD-2) BURLINGTON RESOURCES O & G CO LP 11808 A MUELLER A-KOEHLER SW D EAGLEVILLE (EAGLE FORD-2) BURLINGTON RESOURCES O & G CO LP 11810 A MUELLER UNIT A EAGLEVILLE (EAGLE FORD-2) BURLINGTON RESOURCES O & G CO LP 09796 A MUELLER UNIT B EAGLEVILLE (EAGLE FORD-2) BURLINGTON RESOURCES O & G CO LP 09929 A. -

NZ Penguin Initiative, Q1 2021 Report, Thomas Mattern & Richard
1 NZ Penguin Initiative, Q1 2021 Report, Thomas Mattern & Richard Seed Quarter One (Q1) Report 2021 THOMAS MATTERN (SCIENTIFIC DIRECTOR) RICHARD SEED (RESEARCH & CONSERVATION COORDINATOR) 13TH APRIL 2021 Abstract The transponder gate in Harrison Cove is operational again following nearly a year of technical issues. Comprehensive analysis of three years’ worth of tawaki winter tracking data is underway with plans to publish two scientific papers this winter. Two film production companies plan to work alongside the Tawaki Project this field season; (1) Netflix’s ‘Our Planet’ series and (2) a South American documentary about NZPI advisor Popi Garcia-Borboroglu and the Global Penguin Society. Our comprehensive paper summarizing the survey of Erect-crested penguins on the Bounty Islands has been published in the latest issue of Notornis, while further analysis of drone imagery from the surveys has provided a detailed topographic reconstruction of the area. With the research permits attained, logistics are now be arranged for a 2021 research expedition to the Bounty Islands and Antipodes. Both the NZ Penguin Database and kororā monitoring protocols have been updated following their first year in operation. Establishment and monitoring of a marked kororā population on the West Coast revealed a bird in the colony that was marked as a chick nearly 1000 km round the coast in Otago. Halfmoon bay school have completed the first season of their pilot kororā monitoring project on Rakiura with help from NZPI. The Fiordland community were engaged with our research and penguin conservation through public and school talks. 2 NZ Penguin Initiative, Q1 2021 Report, Thomas Mattern & Richard Seed Contents Winter season of the Tawaki Project ........................................................................................... -

Download Article As 589.6 KB PDF File
6 AvailableNew on-lineZealand at: Journal http://www.newzealandecology.org/nzje/ of Ecology, Vol. 34, No. 1, 2010 special issue: Feathers to Fur The ecological transformation of Aotearoa/New Zealand The origin and history of New Zealand’s terrestrial vertebrates Alan J.D. Tennyson Museum of New Zealand Te Papa Tongarewa, PO Box 467, Wellington, New Zealand (Email: [email protected]) Published on-line: 4 November 2009 Abstract: Since the 1980s, morphological and molecular research has resulted in significant advances in understanding the relationships and origins of the recent terrestrial vertebrate fauna in the New Zealand biogeographic region. This research has led to many taxonomic changes, with a significant increase in the number of bird and reptile species recognised. It has also resulted in the recognition of several more Holocene (<10 000 years ago) bird species extinctions. The conclusion that Holocene extinctions were primarily caused by human- hunting and predation by other introduced mammals (particularly rats and cats) has been supported by new data. Despite many local eradications of introduced pests, the number of introduced species has increased, with the establishment of five more foreign birds and (on Norfolk Island) the house gecko (Hemidactylus frenatus). Many new, significant New Zealand vertebrate fossils have been reported, including more dinosaurs from the Cretaceous, and the first Tertiary records of frogs, rhynchocephalids, lizards, crocodylians, bats and a terrestrial “Mesozoic ghost” mammal from the Early Miocene near St Bathans. For birds, the earliest known penguins in the world have been discovered, and there are intriguing Late Cretaceous – Early Paleocene remains still awaiting detailed description. -

Recent Advances in Avian Palaeobiology in New Zealand with Implications for Understanding New Zealand’S Geological, Climatic and Evolutionary Histories
New Zealand Journal of Zoology ISSN: 0301-4223 (Print) 1175-8821 (Online) Journal homepage: http://www.tandfonline.com/loi/tnzz20 Recent advances in avian palaeobiology in New Zealand with implications for understanding New Zealand’s geological, climatic and evolutionary histories Trevor H. Worthy, Vanesa L. De Pietri & R. Paul Scofield To cite this article: Trevor H. Worthy, Vanesa L. De Pietri & R. Paul Scofield (2017) Recent advances in avian palaeobiology in New Zealand with implications for understanding New Zealand’s geological, climatic and evolutionary histories, New Zealand Journal of Zoology, 44:3, 177-211, DOI: 10.1080/03014223.2017.1307235 To link to this article: https://doi.org/10.1080/03014223.2017.1307235 View supplementary material Published online: 19 Apr 2017. Submit your article to this journal Article views: 239 View related articles View Crossmark data Citing articles: 2 View citing articles Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=tnzz20 NEW ZEALAND JOURNAL OF ZOOLOGY, 2017 VOL. 44, NO. 3, 177–211 https://doi.org/10.1080/03014223.2017.1307235 REVIEW ARTICLE Recent advances in avian palaeobiology in New Zealand with implications for understanding New Zealand’s geological, climatic and evolutionary histories Trevor H. Worthy a, Vanesa L. De Pietri b and R. Paul Scofield b aSchool of Biological Sciences, Flinders University of South Australia, GPO 2100, Adelaide 5001, South Australia; bNatural History Department, Canterbury Museum, Rolleston Avenue, Christchurch 8013, New Zealand ABSTRACT ARTICLE HISTORY New Zealand, long recognised as a land where birds dominate the Received 20 February 2017 terrestrial vertebrate biota, lacked an informative fossil record for Accepted 10 March 2017 the non-marine pre-Pleistocene avifauna until the twenty-first KEYWORDS century. -

Parasitism, Disease and Breeding Ecology of Little Blue Penguins (Eudyptula Minor) on Tiritiri Matangi Island, New Zealand
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere without the permission of the Author. Parasitism, disease and breeding ecology of little blue penguins (Eudyptula minor) on Tiritiri Matangi Island, New Zealand A thesis submitted in partial fulfilment of the requirements for the degree of Master of Science in Conservation Biology Massey University, Auckland. Monique Jansen van Rensburg 2010 “A thing is complete when you can let it be.” Gita Bellin ii ABSTRACT According to the New Zealand Threat Classification, little blue penguin (LBP) (Eudyptula minor ) populations are under ‘gradual decline’. Although longterm data are available for some mainland populations, the status of LBP on offshore islands remains largely unknown. Most studies have focussed on breeding success and foraging ecology. However, there is a paucity of data pertaining to diseases and parasites, and the potential effects of these factors on LBP health, reproductive success and survival. To date, the LBP population on Tiritiri Matangi Island, Hauraki Gulf, Auckland, New Zealand, has only been monitored periodically, despite the island being an important habitat for LBP throughout their annual cycle. The overall aim of this study was to examine the relative importance of parasites and disease in relation to key aspects of LBP life-history, including: the annual cycle; reproductive success; energetic demands, immunity; and mortality. During 2006 and 2007, the reproductive success of LBP on Tiritiri Matangi Island was investigated with respect to lay date, nest site attributes, parental quality and ectoparasite loads. -

Recvolume 29 Rds 2015
RecVolume 29 rds 2015 Records of the Canterbury Museum Records of the Canterbury Museum Volume 29 2015 Contents EDITOR Cor Vink A revision of the distribution maps and database of New Zealand mayflies (Ephemeroptera) 5 at Canterbury Museum EDITORIAL BOARD TERRY R HITCHINGS, TIM R HITCHINGS & MATTHEW D SHAW Roger Fyfe Sarah Murray Harvesting of ngā hua manu (bird eggs) in Te Waipounamu (South Island), New Zealand 35 Paul Scofield ROGER FYFE & KYLE DAVIS Daniel Stirland Anthony Wright Southern Spirits: The Case of the Psychical Research Society of Christchurch 47 JULIAN VESTY & JOANNA COBLEY PRODUCTION Vicki Blyth Instructions for Authors 58 Alex Wootton Canterbury Museum gratefully acknowledges a grant from the Mason Foundation for the publication of the Records of the Canterbury Museum. ISSN 0370 3878 (Print) ISSN 2881 9707 (Online) RECORDS OF THE CANTERBURY MUSEUM Volume 29, 2015 Published September 2015 © Canterbury Museum Rolleston Avenue Christchurch 8013 New Zealand Records of the Canterbury Museum, 2015 Vol. 29: 5–34 © Canterbury Museum 2015 A revision of the distribution maps and database of New Zealand mayflies (Ephemeroptera) at Canterbury Museum TERRY R HITCHINGS Canterbury Museum, Rolleston Avenue, Christchurch 8013, New Zealand [email protected] TIM R HITCHINGS Canterbury Museum, Rolleston Avenue, Christchurch 8013, New Zealand [email protected] MATTHEW D SHAW Canterbury Museum, Rolleston Avenue, Christchurch 8013, New Zealand [email protected] ABSTRACT New Zealand mayflies form an important component of species was given by Wisely (1962). Maps for 30 the freshwater aquatic fauna, being of particular interest Leptophlebiidae distributions were given by Towns and to conservationists, bio-geographers, recreational Peters (1996). -

Bluff Hill Motupohue Super Site Booklet
Super Site for Education Bluff Hill/Motupöhue Scenic Reserve SOUTHLAND CONSERVANCY CONTENTS Introduction 3 Using this resource 3 For teachers 3 What to bring 3 Cross-curricular or specialised 3 Education for the environment 3 Safety 3 Pre and post-visit activities 4 Pre visit activity: be prepared 4 Activity: making the rules 5 Bluff/Motupöhue Hill Scenic Reserve and Töpuni Site 6 Teachers Notes and Activities 7 Geology of Bluff Hill/Motupöhue 7 Bluff Hill - Older than Aotearoa 7 Cooked sediments 7 A whanau of mountains 7 Glossary 7 Activity 1: About the environment (pre-trip) – Bluff Rocks 7 Human Impacts 9 Vanishing birds 9 An empty sea 9 Shrinking forests 9 Draining and dredging 9 Surprising survivors 9 Activity 2: About the environment – the history of the hill 10 Activity 3: In the environment – then and now 11 Significance to Tängata Whenua 12 Toki-toki and Caddell 12 Töpuni – a symbol of protection 12 An ancient name 12 Activity 4: About the environment – history, myth and magic Early European Settlement 13 Small beginnings 13 Early exports 13 Harakeke 14 The essential fibre 14 European trade 14 Activity 5: About the environment. Flax works! 14 1 Whaling from Bluff 15 The rarest whale in the world 15 Rough Bluff 15 Whale oil – the super fuel of its day 15 Right for the wrong reasons 15 Whales welcome 15 Keeping fish and chips on the menu 15 For us and our children after us 16 Activity 6: For the environment – a waste of whales 16 Activity 7: In the environment/for the environment – boat versus whale 16 Bluff Coastal Defence Camp 17 Activity 8: In the environment/for the environment 17 The Walk 18 Coastal vegetation – each plant in its place 18 Activity 9: About the environment – what do you know? 19 Activity 10: Leaf bingo 19 Activity 11: In the environment: You’re the expert 19 Activity 12: In the environment – Poet-tree 20 Appendix 1: Activities 21 Contacts 39 Resources 39 History 39 Links 39 2 Introduction Using this resource SUPER SITES © are designed to give you a hand to plan exciting and educational learning experiences outside the classroom. -

04/28/2021 Railroad Commission of Texas Page 1 Oil Leases
(OGPFS) 04/28/2021 RAILROAD COMMISSION OF TEXAS PAGE 1 OIL LEASES AND GAS WELLS BY DISTRICT, OPERATOR, OIL/GAS, LEASE NAME DIST OPER # OPERATOR NAME O/G LSE/ID LEASE NAME FIELD # FIELD NAME CNTY# COUNTY NAME 02 000017 A + INVESTMENTS A TEXAS LLC GAS 140659 EL PASO NATURAL GAS 16970425 CHAPA, EAST (9500) 297 LIVE OAK GAS 172853 EL PASO NATURAL GAS UNIT 16970750 CHAPA, EAST (12,400) 297 LIVE OAK 02 000202 A & D EXPLORATION COMPANY GAS 176165 STOLDT 03005800 ANNA BARRE, N. (YEGUA 5260) 123 DE WITT 02 000234 A & G OIL PRODUCTION CO. OIL 02002 GATES, BERTHA M. 26692750 DUNWOLTERS (SEG. D) 255 KARNES 02 000253 A.J.& M. OIL COMPANY OIL 07598 BUTLER, M.R. 21048001 COTTONWOOD CREEK, SOUTH 123 DE WITT 02 000555 A.O.E. OPERATORS, INC. OIL 00635 HAMILTON FEE 40239304 HELEN GOHLKE, W. (WILCOX) 469 VICTORIA 02 000578 A & R RESOURCES GAS 151587 PARKS 30178103 FANNIN, SOUTH (TOBY) 175 GOLIAD 02 000894 ABACO OPERATING, LLC OIL 11219 BURDITT, ALLEN L. 51109306 LA WARD, NORTH (PIGPEN SAND) 239 JACKSON OIL 00792 BURDITT, ALLEN L. 51109001 LA WARD, NORTH 239 JACKSON GAS 184658 BURDITT, ALLEN L. 51109324 LA WARD, NORTH (4700) 239 JACKSON 02 003399 ACCIDIE EXPLORATION AND DEV.,INC OIL 01119 TRAYLOR, MAUDE B., MRS. -A- 67197750 OLIVIA (8300) 057 CALHOUN 02 003596 ACOCK OPERATING LIMITED OIL 09121 PROBST, DOROTHY 74943500 RAY POINT SW. (WILCOX) 297 LIVE OAK OIL 07132 PROBST, DOROTHY 74943400 RAY POINT, SW. (SLICK-WILCOX) 297 LIVE OAK OIL 05245 PROBST, DOROTHY 74943500 RAY POINT SW. (WILCOX) 297 LIVE OAK GAS 162985 E. -

John Cockrem – Penguins and Birds
BEFORE THE ENVIRONMENTAL PROTECTION AUTHORITY AT WELLINGTON IN THE MATTER of the Exclusive Economic Zone and Continental Shelf (Environmental Effects) Act 2012 (“the Act”) AND IN THE MATTER of the applications by Trans Tasman Resources Limited (TTR) for marine and discharge consents to recover iron sand under sections 20 and 87B of the Act and BETWEEN Trans-Tasman Resources Limited Applicant AND The Environmental Protection Authority EPA AND Kiwis Against Seabed Mining Incorporated (KASM) Submitter STATEMENT OF EVIDENCE BY JOHN COCKREM ON BEHALF OF KIWIS AGAINST SEABED MINING INCORPORATED Dated 23 January 2017 Duncan Currie/Ruby Haazen 8 Mt Eden Road Eden Terrace AUCKLAND [email protected] Ph: 021-144-3457 Evidence of Dr John Cockrem STATEMENT OF EVIDENCE OF DR JOHN COCKREM INTRODUCTION 1 My name is John Fenton Cockrem. 2 I hold a BSc(Hons) from Massey University and a PhD from the University of Bristol. I am Professor of Comparative Endocrinology at Massey University, Palmerston North. 3 I have conducted research on birds and other animals for more than 35 years. I have studied Adelie, emperor, yellow-eyed and little penguins, and a wide range of other species including amphibians, reptiles, birds, and marine and terrestrial mammals. I have published 96 refereed journal articles and 5 refereed book chapters, and have 150 other publications. I have 1825 citations in Web of Science, with my two most highly cited first author papers having 111 and 109 citations. I have been a visiting scientist in the United Kingdom, Sweden, Japan and the United States. International consultancy work has been performed for the United States Navy Office of Naval Research and for the National Commission for Wildlife Conservation and Development in Saudi Arabia. -

Legal Protection of New Zealand's Indigenous Terrestrial Fauna
Tuhinga 25: 25–101 Copyright © Museum of New Zealand Te Papa Tongarewa (2014) Legal protection of New Zealand’s indigenous terrestrial fauna – an historical review Colin M. Miskelly Museum of New Zealand Te Papa Tongarewa, PO Box 467, Wellington, New Zealand ([email protected]) ABSTRACT: New Zealand has had a complex history of wildlife protection, with at least 609 different pieces of legislation affecting the protection of native wildlife between 1861 and 2013. The first species to be fully protected was the tüï (Prosthemadera novaeseelandiae), which was listed as a native game species in 1873 and excluded from hunting in all game season notices continuously from 1878, until being absolutely protected in 1906. The white heron (Ardea modesta) and crested grebe (Podiceps cristatus) were similarly protected nationwide from 1888, and the huia (Heteralocha acutirostris) from 1892. Other species listed as native game before 1903 were not consistently excluded from hunting in game season notices, meaning that such iconic species as kiwi (Apteryx spp.), käkäpö (Strigops habroptilus), kökako (Callaeas spp.), saddlebacks (Philesturnus spp.), stitchbird (Notiomystis cincta) and bellbird (Anthornis melanura) could still be taken or killed during the game season until they were absolutely protected in 1906. The tuatara (Sphenodon punctatus) was added to the native game list in 1895, but due to inadequate legislation was not absolutely protected until 1907. The Governor of the Colony of New Zealand had the power to absolutely protect native birds from 1886, but this was not used until 1903, when first the blue duck (Hymenolaimus malacorhynchus) and then the huia were given the status of absolutely protected, followed by more than 130 bird species by the end of 1906. -
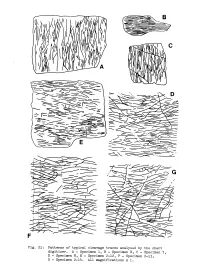
Patterns of Typical Cleavage Traces Analysed by the Chart Digitizer. A
• • Fig. 21: Patterns of typical cleavage traces analysed by the chart digitizer. A - Specimen 1, B - Specimen B, C - Specimen 7, D - Specimen 8, E - Specimen 2-12, F - Specimen 2-13, G - Specimen 2-14. All magnifications x 1. 34. electronic chart digitizer at the Division of Mineral Physics, CSIRO, North Ryde, and results calculated by program GOLLUM (see Appendix II). Initially two specimens were digitised (Specimen A, Plate 5A, and Specimen B, Fig.21B) and results for lengths and spacings are shown in Figure 22. On graphs of length versus orientation from the dominant cleavage plane (Figs. 23A and B) the pattern as predicted by the model in Chapter 1 (p.25-29) was not observed. Instead, a distribution to both sides of the dominant cleavage plane occurred. The real distributions exhibit a close similarity to the theoretical curves, indicating that the cleavage planes are diverging on both sides away from the dominant cleavage plane. Because of the unusual pattern encountered it was decided to examine more specimens, particularly of "fracture" cleavage to see if similar patterns could be recognised. Specimen B for slaty cleavage has an almost parallel orientation with only two cleavage planes out of a total of 66 diverging more than 8° from the dominant cleavage plane. Subsequently the lengths and orientations of cleavage planes in fourteen specimens and photographs were determined using the electronic digitizer. Specimens were collected from four main localities within the Block and can be grouped into five divisions with respect to the orientation of the bedding and mesoscopic folds: (a) the plane of the specimen is parallel to the ac-plane, and bedding and the dominent cleavage are subvertical (e.g.