Status, Trends, and Future Dynamics of Freshwater Ecosystems in Europe and Central Asia R
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cfreptiles & Amphibians
WWW.IRCF.ORG TABLE OF CONTENTS IRCF REPTILES &IRCF AMPHIBIANS REPTILES • VOL &15, AMPHIBIANS NO 4 • DEC 2008 • 189 27(2):288–292 • AUG 2020 IRCF REPTILES & AMPHIBIANS CONSERVATION AND NATURAL HISTORY TABLE OF CONTENTS FEATURE ARTICLES . Chasing BullsnakesAmphibians (Pituophis catenifer sayi) in Wisconsin: of the Melghat, On the Road to Understanding the Ecology and Conservation of the Midwest’s Giant Serpent ...................... Joshua M. Kapfer 190 . The Shared History of TreeboasMaharashtra, (Corallus grenadensis) and Humans on Grenada: India A Hypothetical Excursion ............................................................................................................................Robert W. Henderson 198 RESEARCH ARTICLES Hayat A. Qureshi and Gajanan A. Wagh . Biodiversity Research Laboratory,The Texas Horned Department Lizard in of Central Zoology, and ShriWestern Shivaji Texas Science ....................... College, Emily Amravati, Henry, Jason Maharashtra–444603, Brewer, Krista Mougey, India and Gad (gaj [email protected]) 204 . The Knight Anole (Anolis equestris) in Florida .............................................Brian J. Camposano,Photographs Kenneth L. Krysko, by the Kevin authors. M. Enge, Ellen M. Donlan, and Michael Granatosky 212 CONSERVATION ALERT . World’s Mammals in Crisis ............................................................................................................................................................. 220 . More Than Mammals ..................................................................................................................................................................... -

Bibliography and Scientific Name Index to Amphibians
lb BIBLIOGRAPHY AND SCIENTIFIC NAME INDEX TO AMPHIBIANS AND REPTILES IN THE PUBLICATIONS OF THE BIOLOGICAL SOCIETY OF WASHINGTON BULLETIN 1-8, 1918-1988 AND PROCEEDINGS 1-100, 1882-1987 fi pp ERNEST A. LINER Houma, Louisiana SMITHSONIAN HERPETOLOGICAL INFORMATION SERVICE NO. 92 1992 SMITHSONIAN HERPETOLOGICAL INFORMATION SERVICE The SHIS series publishes and distributes translations, bibliographies, indices, and similar items judged useful to individuals interested in the biology of amphibians and reptiles, but unlikely to be published in the normal technical journals. Single copies are distributed free to interested individuals. Libraries, herpetological associations, and research laboratories are invited to exchange their publications with the Division of Amphibians and Reptiles. We wish to encourage individuals to share their bibliographies, translations, etc. with other herpetologists through the SHIS series. If you have such items please contact George Zug for instructions on preparation and submission. Contributors receive 50 free copies. Please address all requests for copies and inquiries to George Zug, Division of Amphibians and Reptiles, National Museum of Natural History, Smithsonian Institution, Washington DC 20560 USA. Please include a self-addressed mailing label with requests. INTRODUCTION The present alphabetical listing by author (s) covers all papers bearing on herpetology that have appeared in Volume 1-100, 1882-1987, of the Proceedings of the Biological Society of Washington and the four numbers of the Bulletin series concerning reference to amphibians and reptiles. From Volume 1 through 82 (in part) , the articles were issued as separates with only the volume number, page numbers and year printed on each. Articles in Volume 82 (in part) through 89 were issued with volume number, article number, page numbers and year. -

Three New Species of Alburnoides (Teleostei: Cyprinidae) from Euphrates River, Eastern Anatolia, Turkey
Zootaxa 3754 (2): 101–116 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2014 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3754.2.1 http://zoobank.org/urn:lsid:zoobank.org:pub:33DCB673-BC7C-4DB2-84CE-5AC5C6AD2052 Three new species of Alburnoides (Teleostei: Cyprinidae) from Euphrates River, Eastern Anatolia, Turkey DAVUT TURAN1,3, CÜNEYT KAYA1, F. GÜLER EKMEKÇİ2 & ESRA DOĞAN1 1Recep Tayyip Erdoğan University, Faculty of Fisheries and Aquatic Sciences, 53100 Rize, Turkey. E-mail: [email protected]; [email protected]; [email protected] 2Department of Biology, Faculty of Sciences, Hacettepe University, Beytepe Campus, 06800 Ankara, Turkey. E-mail: [email protected] 3Corresponding author. E-mail: [email protected] Abstract Three new species of Alburnoides, Alburnoides emineae sp. n., Alburnoides velioglui sp. n., Alburnoides recepi sp. n., are described from the Euphrates River drainages (Persian Gulf basin) in eastern Anatolia, Turkey. Alburnoides emineae, from Beyazsu Stream (south-eastern Euphrates River drainage), is distinguished from all species of Alburnoides in Turkey and adjacent regions by a combination of the following characters (none unique to the species): a well developed ventral keel between pelvic and anal fins, commonly scaleless or very rarely 1–2 scales covering the anterior portion of the keel; a deep body (depth at dorsal-fin origin 31–36% SL); 37–43 + 1–2 lateral-line scales, 13½–15½ branched anal-fin rays; number of total vertebrae 41–42, modally 41, comprising 20–21 abdominal and 20–21 caudal vertebrae. Alburnoides velioglui, from Sırlı, Karasu, Divriği and Sultansuyu streams (northern and northeastern Euphrates River drainages), is distinguished by a poorly developed ventral keel, completely scaled; a moderately deep body (depth at dorsal-fin origin 24–29% SL); 45–53 + 1–2 lateral-line scales, 11½ –13½ branched anal-fin rays; number of total vertebrae 41–42, modally 42, comprising 20–22 abdominal and 20–21 caudal vertebrae. -

The IUCN Red List of Threatened Speciestm
Species 2014 Annual ReportSpecies the Species of 2014 Survival Commission and the Global Species Programme Species ISSUE 56 2014 Annual Report of the Species Survival Commission and the Global Species Programme • 2014 Spotlight on High-level Interventions IUCN SSC • IUCN Red List at 50 • Specialist Group Reports Ethiopian Wolf (Canis simensis), Endangered. © Martin Harvey Muhammad Yazid Muhammad © Amazing Species: Bleeding Toad The Bleeding Toad, Leptophryne cruentata, is listed as Critically Endangered on The IUCN Red List of Threatened SpeciesTM. It is endemic to West Java, Indonesia, specifically around Mount Gede, Mount Pangaro and south of Sukabumi. The Bleeding Toad’s scientific name, cruentata, is from the Latin word meaning “bleeding” because of the frog’s overall reddish-purple appearance and blood-red and yellow marbling on its back. Geographical range The population declined drastically after the eruption of Mount Galunggung in 1987. It is Knowledge believed that other declining factors may be habitat alteration, loss, and fragmentation. Experts Although the lethal chytrid fungus, responsible for devastating declines (and possible Get Involved extinctions) in amphibian populations globally, has not been recorded in this area, the sudden decline in a creekside population is reminiscent of declines in similar amphibian species due to the presence of this pathogen. Only one individual Bleeding Toad was sighted from 1990 to 2003. Part of the range of Bleeding Toad is located in Gunung Gede Pangrango National Park. Future conservation actions should include population surveys and possible captive breeding plans. The production of the IUCN Red List of Threatened Species™ is made possible through the IUCN Red List Partnership. -

Reconstruction of Oligocene and Neogene Freshwater Fish Faunas—An Actualistic Study on Cypriniform Otoliths
Reconstruction of Oligocene and Neogene freshwater fish faunas—an actualistic study on cypriniform otoliths TANJA SCHULZ−MIRBACH and BETTINA REICHENBACHER Schulz−Mirbach, T. and Reichenbacher, B. 2006. Reconstruction of Oligocene and Neogene freshwater fish faunas—an actualistic study on cypriniform otoliths. Acta Palaeontologica Polonica 51 (2): 283–304. Fossil utricular otoliths (= lapilli) from cypriniform fishes have long been recorded from European Oligocene and Neo− gene freshwater and oligohaline sediments. Until now, their determination was limited to the family level owing to the lack of morphological investigations on lapilli of Recent cypriniforms. The present study introduces a terminology for the lapillus morphology that is based on the lapilli of 134 specimens of 20 cyprinid and one balitorid species. It is demon− strated that the lapillus has valuable characters for taxonomic classification. As a result, fossil lapilli from Oligocene and Miocene continental deposits from the western Mediterranean, the Swiss and the South German Molasse Basin, the Mainz Basin, and additionally from Anatolia could be determined. Nine species were identified: aff. Abramis sp. vel aff. Alburnus sp., aff. Alburnoides sp., aff. Barbus sp., cf. Leuciscus sp., Palaeoleuciscus sp., Palaeotinca moeddeni sp. nov., Palaeotinca sp. 1, aff. Phoxinus sp., and aff. Rutilus sp. vel aff. Scardinius sp. Our study includes the oldest record of a Phoxinus−related and a Palaeotinca species from Europe. Additionally, aff. Abramis sp. vel aff. Alburnus sp. and aff. Alburnoides have been identified as fossils for the first time. The determination of the fossil lapilli has been supported by means of pharyngeal teeth, with the exception of aff. Abramis sp. -

Review of Fisheries and Aquaculture Development Potentials in Georgia
FAO Fisheries and Aquaculture Circular No. 1055/1 REU/C1055/1(En) ISSN 2070-6065 REVIEW OF FISHERIES AND AQUACULTURE DEVELOPMENT POTENTIALS IN GEORGIA Copies of FAO publications can be requested from: Sales and Marketing Group Office of Knowledge Exchange, Research and Extension Food and Agriculture Organization of the United Nations E-mail: [email protected] Fax: +39 06 57053360 Web site: www.fao.org/icatalog/inter-e.htm FAO Fisheries and Aquaculture Circular No. 1055/1 REU/C1055/1 (En) REVIEW OF FISHERIES AND AQUACULTURE DEVELOPMENT POTENTIALS IN GEORGIA by Marina Khavtasi † Senior Specialist Department of Integrated Environmental Management and Biodiversity Ministry of the Environment Protection and Natural Resources Tbilisi, Georgia Marina Makarova Head of Division Water Resources Protection Ministry of the Environment Protection and Natural Resources Tbilisi, Georgia Irina Lomashvili Senior Specialist Department of Integrated Environmental Management and Biodiversity Ministry of the Environment Protection and Natural Resources Tbilisi, Georgia Archil Phartsvania National Consultant Thomas Moth-Poulsen Fishery Officer FAO Regional Office for Europe and Central Asia Budapest, Hungary András Woynarovich FAO Consultant FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 2010 The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned. -

Europe's Biodiversity
EEA Europe’s Biodiversity, Steppic region. 2006-05-02 page 1 European Environment Agency Europe’s biodiversity – biogeographical regions and seas Biogeographical regions in Europe The Steppic Region – the plains of Europe Original contributions from ETC/NPB: Sophie Condé, Dominique Richard (coordinators) Nathalie Liamine (editor) Anne-Sophie Leclère (data collection and processing) Barbara Sotolargo (drafting) Ulla Pinborg (final co-editor) Map production: UNEP/GRID Warsaw (final production) Project manager: Tor-Björn Larsson, EEA ZooBoTech HB, Sweden, Linus Svensson (final edition) EEA Europe’s Biodiversity, Steppic region. 2006-05-02 page 2 Biogeographical regions in Europe .................................................................. 1 – the plains of Europe ..................................................................................... 1 Original contributions from ETC/NPB: ............................................................... 1 Map production: ............................................................................................ 1 Summary ............................................................................................................ 3 1. What are the main characteristics and trends of the Steppic biogeographical region? ............................................................................................................... 3 1.1 General characteristics.............................................................................. 3 1.1.1 Extent and boundaries .......................................................................... -

AMPHIBIA: ANURA: LEPTODACTYLIDAE Leptodactylus Pentadactylus
887.1 AMPHIBIA: ANURA: LEPTODACTYLIDAE Leptodactylus pentadactylus Catalogue of American Amphibians and Reptiles. Heyer, M.M., W.R. Heyer, and R.O. de Sá. 2011. Leptodactylus pentadactylus . Leptodactylus pentadactylus (Laurenti) Smoky Jungle Frog Rana pentadactyla Laurenti 1768:32. Type-locality, “Indiis,” corrected to Suriname by Müller (1927: 276). Neotype, Nationaal Natuurhistorisch Mu- seum (RMNH) 29559, adult male, collector and date of collection unknown (examined by WRH). Rana gigas Spix 1824:25. Type-locality, “in locis palu - FIGURE 1. Leptodactylus pentadactylus , Brazil, Pará, Cacho- dosis fluminis Amazonum [Brazil]”. Holotype, Zoo- eira Juruá. Photograph courtesy of Laurie J. Vitt. logisches Sammlung des Bayerischen Staates (ZSM) 89/1921, now destroyed (Hoogmoed and Gruber 1983). See Nomenclatural History . Pre- lacustribus fluvii Amazonum [Brazil]”. Holotype, occupied by Rana gigas Wallbaum 1784 (= Rhin- ZSM 2502/0, now destroyed (Hoogmoed and ella marina {Linnaeus 1758}). Gruber 1983). Rana coriacea Spix 1824:29. Type-locality: “aquis Rana pachypus bilineata Mayer 1835:24. Type-local MAP . Distribution of Leptodactylus pentadactylus . The locality of the neotype is indicated by an open circle. A dot may rep - resent more than one site. Predicted distribution (dark-shaded) is modified from a BIOCLIM analysis. Published locality data used to generate the map should be considered as secondary sources, as we did not confirm identifications for all specimen localities. The locality coordinate data and sources are available on a spread sheet at http://learning.richmond.edu/ Leptodactylus. 887.2 FIGURE 2. Tadpole of Leptodactylus pentadactylus , USNM 576263, Brazil, Amazonas, Reserva Ducke. Scale bar = 5 mm. Type -locality, “Roque, Peru [06 o24’S, 76 o48’W].” Lectotype, Naturhistoriska Riksmuseet (NHMG) 497, age, sex, collector and date of collection un- known (not examined by authors). -

Amphibian Background
The Toledo Zoo/ThinkingWorks Teacher Overview for the Amphibian Lessons Ó2003 Teacher Overview: Amphibians Amphibians have many traits that are unique to this particular class of animals. Below is a list of general amphibian traits to help you and your students complete the ThinkingWorks lesson. The class Amphibia is divided into three groups or orders, each with their own set of features. The orders are frogs and toads, salamanders and caecilians. We have included a list of the different amphibians found at The Toledo Zoo by order and where you can find them on exhibit. Note that animals move constantly in and out of the Zoo. Please call the Zoo for a current list of amphibians that are on exhibit and their locations. Wild toad tadpoles and adults can also be observed on Zoo grounds in the formal garden area near the Conservatory. Look near the pool in the butterfly garden. General Amphibian Traits q The life cycle of an amphibian (the name means “two-lived”) begins as an egg. Tadpoles or larvae (singular is larva) hatch from the egg. Tadpoles are an immature stage. The tadpoles then mature into adults. This process is called metamorphosis. q Females deposit eggs in water where they are fertilized externally by males (see diagram). q Eggs hatch into tadpoles that are aquatic (live in water), breathe through gills instead of lungs, have a tail, no eyelids, ears and, initially, no legs. Adult Male Tadpole (Larva) Adult Female Eggs q Adults of most amphibians have four legs, lungs, eyelids, tear glands and ears. q Besides lungs, most adult amphibians can exchange gases through the skin and the membranes lining the mouth. -
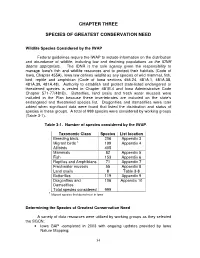
Chapter Three Species of Greatest Conservation Need
CHAPTER THREE SPECIES OF GREATEST CONSERVATION NEED Wildlife Species Considered by the IWAP Federal guidelines require the IWAP to include information on the distribution and abundance of wildlife, including low and declining populations as the IDNR deems appropriate. The IDNR is the sole agency given the responsibility to manage Iowa's fish and wildlife resources and to protect their habitats (Code of Iowa, Chapter 455A). Iowa law defines wildlife as any species of wild mammal, fish, bird, reptile and amphibian (Code of Iowa sections 456.24, 481A.1, 481A.38, 481A.39, 481A.48). Authority to establish and protect state-listed endangered or threatened species is vested in Chapter 481B.4 and Iowa Administrative Code Chapter 571-77(481B). Butterflies, land snails and fresh water mussels were included in the Plan because these invertebrates are included on the state’s endangered and threatened species list. Dragonflies and damselflies were later added when significant data were found that listed the distribution and status of species in these groups. A total of 999 species were considered by working groups (Table 3-1). Table 3-1. Number of species considered by the IWAP . Taxonomic Class Species List location Breeding birds 206 Appendix 3 Migrant birds 1 199 Appendix 4 All birds 405 Mammals 82 Appendix 5 Fish 153 Appendix 6 Reptiles and Amphibians 71 Appendix 7 Freshwater mussels 55 Appendix 8 Land snails 8 Table 3-8 Butterflies 119 Appendix 9 Dragonflies and 106 Appendix 10 Damselflies Total species considered 999 1 Migrant species that do -

Checklist of Amphibians, Reptiles, Birds and Mammals of New York
CHECKLIST OF AMPHIBIANS, REPTILES, BIRDS AND MAMMALS OF NEW YORK STATE Including Their Legal Status Eastern Milk Snake Moose Blue-spotted Salamander Common Loon New York State Artwork by Jean Gawalt Department of Environmental Conservation Division of Fish and Wildlife Page 1 of 30 February 2019 New York State Department of Environmental Conservation Division of Fish and Wildlife Wildlife Diversity Group 625 Broadway Albany, New York 12233-4754 This web version is based upon an original hard copy version of Checklist of the Amphibians, Reptiles, Birds and Mammals of New York, Including Their Protective Status which was first published in 1985 and revised and reprinted in 1987. This version has had substantial revision in content and form. First printing - 1985 Second printing (rev.) - 1987 Third revision - 2001 Fourth revision - 2003 Fifth revision - 2005 Sixth revision - December 2005 Seventh revision - November 2006 Eighth revision - September 2007 Ninth revision - April 2010 Tenth revision – February 2019 Page 2 of 30 Introduction The following list of amphibians (34 species), reptiles (38), birds (474) and mammals (93) indicates those vertebrate species believed to be part of the fauna of New York and the present legal status of these species in New York State. Common and scientific nomenclature is as according to: Crother (2008) for amphibians and reptiles; the American Ornithologists' Union (1983 and 2009) for birds; and Wilson and Reeder (2005) for mammals. Expected occurrence in New York State is based on: Conant and Collins (1991) for amphibians and reptiles; Levine (1998) and the New York State Ornithological Association (2009) for birds; and New York State Museum records for terrestrial mammals. -

Systematics of Leptopelis (Anura: Arthroleptidae) from the Itombwe
University of Texas at El Paso DigitalCommons@UTEP Open Access Theses & Dissertations 2012-01-01 Systematics of Leptopelis (Anura: Arthroleptidae) from the Itombwe Plateau, Eastern Democratic Republic of the Congo Francisco Portillo University of Texas at El Paso, [email protected] Follow this and additional works at: https://digitalcommons.utep.edu/open_etd Part of the Biology Commons, Developmental Biology Commons, Evolution Commons, and the Zoology Commons Recommended Citation Portillo, Francisco, "Systematics of Leptopelis (Anura: Arthroleptidae) from the Itombwe Plateau, Eastern Democratic Republic of the Congo" (2012). Open Access Theses & Dissertations. 1906. https://digitalcommons.utep.edu/open_etd/1906 This is brought to you for free and open access by DigitalCommons@UTEP. It has been accepted for inclusion in Open Access Theses & Dissertations by an authorized administrator of DigitalCommons@UTEP. For more information, please contact [email protected]. SYSTEMATICS OF LEPTOPELIS (ANURA: ARTHROLEPTIDAE) FROM THE ITOMBWE PLATEAU, EASTERN DEMOCRATIC REPUBLIC OF THE CONGO FRANK PORTILLO Department of Biological Sciences APPROVED: ______________________________ Eli Greenbaum, Ph.D., Chair ______________________________ Jerry D. Johnson, Ph.D. ______________________________ Rip Langford, Ph.D. ______________________________________ Benjamin C. Flores, Ph.D. Dean of the Graduate School Copyright © by Frank Portillo 2012 SYSTEMATICS OF LEPTOPELIS (ANURA: ARTHROLEPTIDAE) FROM THE ITOMBWE PLATEAU, EASTERN DEMOCRATIC REPUBLIC OF THE CONGO by FRANK PORTILLO, B.S. THESIS Presented to the Faculty of the Graduate School of The University of Texas at El Paso in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE Department of Biological Sciences THE UNIVERSITY OF TEXAS AT EL PASO December 2012 ACKNOWLEDGMENTS First I would like to thank my family for their love and support throughout my life.