Daily Controlled Physiotherapy Increases Survival Time in Dogs with Suspected Degenerative Myelopathy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dog Breeds of the World
Dog Breeds of the World Get your own copy of this book Visit: www.plexidors.com Call: 800-283-8045 Written by: Maria Sadowski PlexiDor Performance Pet Doors 4523 30th St West #E502 Bradenton, FL 34207 http://www.plexidors.com Dog Breeds of the World is written by Maria Sadowski Copyright @2015 by PlexiDor Performance Pet Doors Published in the United States of America August 2015 All rights reserved. No portion of this book may be reproduced or transmitted in any form or by any electronic or mechanical means, including photocopying, recording, or by any information retrieval and storage system without permission from PlexiDor Performance Pet Doors. Stock images from canstockphoto.com, istockphoto.com, and dreamstime.com Dog Breeds of the World It isn’t possible to put an exact number on the Does breed matter? dog breeds of the world, because many varieties can be recognized by one breed registration The breed matters to a certain extent. Many group but not by another. The World Canine people believe that dog breeds mostly have an Organization is the largest internationally impact on the outside of the dog, but through the accepted registry of dog breeds, and they have ages breeds have been created based on wanted more than 340 breeds. behaviors such as hunting and herding. Dog breeds aren’t scientifical classifications; they’re It is important to pick a dog that fits the family’s groupings based on similar characteristics of lifestyle. If you want a dog with a special look but appearance and behavior. Some breeds have the breed characterics seem difficult to handle you existed for thousands of years, and others are fairly might want to look for a mixed breed dog. -

Dog Breeds in Groups
Dog Facts: Dog Breeds & Groups Terrier Group Hound Group A breed is a relatively homogeneous group of animals People familiar with this Most hounds share within a species, developed and maintained by man. All Group invariably comment the common ancestral dogs, impure as well as pure-bred, and several wild cousins on the distinctive terrier trait of being used for such as wolves and foxes, are one family. Each breed was personality. These are feisty, en- hunting. Some use created by man, using selective breeding to get desired ergetic dogs whose sizes range acute scenting powers to follow qualities. The result is an almost unbelievable diversity of from fairly small, as in the Nor- a trail. Others demonstrate a phe- purebred dogs which will, when bred to others of their breed folk, Cairn or West Highland nomenal gift of stamina as they produce their own kind. Through the ages, man designed White Terrier, to the grand Aire- relentlessly run down quarry. dogs that could hunt, guard, or herd according to his needs. dale Terrier. Terriers typically Beyond this, however, generali- The following is the listing of the 7 American Kennel have little tolerance for other zations about hounds are hard Club Groups in which similar breeds are organized. There animals, including other dogs. to come by, since the Group en- are other dog registries, such as the United Kennel Club Their ancestors were bred to compasses quite a diverse lot. (known as the UKC) that lists these and many other breeds hunt and kill vermin. Many con- There are Pharaoh Hounds, Nor- of dogs not recognized by the AKC at present. -

Dogo Argentino, Fila Brasileiro, Broholmer, Deutscher Boxer
CACIB DRACULA Special CAC 1 CAC 2 Calificare Crufts 13.09.2014 13.09.2014 13.09.2014 Judge //////////////////////////////////// ///////////////////////////////////// ///////////////////////////////// Special CAC 3 CAC 4 CACIB 14.09.2014 14.09.2014 CHAMPIONSHIP 14.09.2014 Schnauzers, Tosa, Cão Fila Dogo Argentino, Fila de São Miguel, Cão Brasileiro, Broholmer, da Serra da Estrela, Cão de Deutscher Boxer, Castro Laboreiro, Rafeiro Mastin español, do Alentejo, Grosser Mastin del Pirineo, St. Schweizer Sennenhund, Bernhardshund, Entlebucher Sennenhund, dr.Molnar Zsolt Russkyi Tchiorny Dobermann, Pinschers, /////////////////////////////////////// Terrier, Dogue de Hollandse Smoushound, Gr.I Dagmar Klein Bordeaux, Bulldog, Cane Corso, Coban Hovawart, Leonberger, Köpegi, Jugosloveski Chien de Montagne Ovcarsky Pas-Sarplaninac, des Pyrenees, Chien de Montagne de Appenzeller I'Atlas (Aïdi), Kraski Sennenhund, Ovcar BOG I BOG II Schnauzers, Tosa, Cão Fila de São Miguel, Shar Pei, Deutsche Cão da Serra da Dogge, Dogo Argentino, Fila Estrela, Cão de Castro Mastino Napoletano, Brasileiro, Broholmer, Laboreiro, Rafeiro do Landseer, Deutscher Boxer, Mastin Alentejo, Grosser Kavkazskaïa Ovtcharka, español, Mastin del Schweizer Sredneasiatskaïa Pirineo, St. Sennenhund, Liz-Beth C. Liljeqvist Ovtcharka, Bernhardshund, Russkyi Entlebucher //////////////////////////////////////// Newfoundland, Berner Tchiorny Terrier, Dogue de Sennenhund, Alexey Belkyn Sennenhund ,Rottweiler, Bordeaux, Bulldog, Dobermann, Perro dogo Mallorquin Hovawart, Leonberger, Pinschers, -

Quiz Sheets BDB JWB 0513.Indd
2. A-Z Dog Breeds Quiz Write one breed of dog for each letter of the alphabet and receive one point for each correct answer. There’s an extra five points on offer for those that can find correct answers for Q, U, X and Z! A N B O C P D Q E R F S G T H U I V J W K X L Y M Z Dogs for the Disabled The Frances Hay Centre, Blacklocks Hill, Banbury, Oxon, OX17 2BS Tel: 01295 252600 www.dogsforthedisabled.org supporting Registered Charity No. 1092960 (England & Wales) Registered in Scotland: SCO 39828 ANSWERS 2. A-Z Dog Breeds Quiz Write a breed of dog for each letter of the alphabet (one point each). Additional five points each if you get the correct answers for letters Q, U, X or Z. Or two points each for the best imaginative breed you come up with. A. Curly-coated Retriever I. P. Tibetan Spaniel Affenpinscher Cuvac Ibizan Hound Papillon Tibetan TerrieR Afghan Hound Irish Terrier Parson Russell Terrier Airedale Terrier D Irish Setter Pekingese U. Akita Inu Dachshund Irish Water Spaniel Pembroke Corgi No Breed Found Alaskan Husky Dalmatian Irish Wolfhound Peruvian Hairless Dog Alaskan Malamute Dandie Dinmont Terrier Italian Greyhound Pharaoh Hound V. Alsatian Danish Chicken Dog Italian Spinone Pointer Valley Bulldog American Bulldog Danish Mastiff Pomeranian Vanguard Bulldog American Cocker Deutsche Dogge J. Portugese Water Dog Victorian Bulldog Spaniel Dingo Jack Russel Terrier Poodle Villano de Las American Eskimo Dog Doberman Japanese Akita Pug Encartaciones American Pit Bull Terrier Dogo Argentino Japanese Chin Puli Vizsla Anatolian Shepherd Dogue de Bordeaux Jindo Pumi Volpino Italiano Dog Vucciriscu Appenzeller Moutain E. -

Memoria 2020
Memoria ejercicio 2020 RIVIA AÑO MEMORIA RIVIA 2020 RIVIA Memoria ejercicio 2020 Los datos publicados pertenecen al Registro Supramunicipal de Animales de Compañía, creado por Decreto 158/1996, de 13 de agosto, del Gobierno Valenciano, por el que se desarrolla la Ley de la Generalitat Valenciana 4/1994, de 8 de julio sobre Protección de los Animales de Compañía, titularidad de la Conselleria de Agricultura, Desarrollo Rural, Emergencia Climática y Transición Ecológica. ADVERTENCIA: Si usted hace uso de los datos de esta Memoria, deberá quedar constancia en las publicaciones y uso la procedencia de los mismos. Febrero 2020 RIVIA Memoria ejercicio 2020 ÉQUIDOS ÉQUIDOS DE ALTA A 31/12/2020 EN LA COMUNITAT VALENCIANA Especie ALICANTE CASTELLON VALENCIA Total Caballo 5892 3757 9012 18661 Asno 881 289 801 1971 Mulo 157 126 169 452 Cebra 19 18 37 Burdégano 12 10 24 46 Total 6961 4182 10024 21167 ÉQUIDOS IDENTIFICADOS POR EL RIVIA EN 2020 EN LA COMUNITAT VALENCIANA Especie y prototipo racial ALICANTE CASTELLON VALENCIA Total Caballo 187 121 275 583 Apaloosa 1 1 2 Asturcón 1 1 Belgian Warmblood 2 2 Caballo deporte Español 1 4 1 6 Caballo deporte portugués 1 1 2 Cavall Pirinenc Català 3 3 Conjunto Mestizo 126 79 193 398 Falabella 2 1 3 Frisona 1 1 Hispano-Árabe 2 6 2 10 Hispano-Bretón 1 4 5 10 Jaca Navarra 1 1 Percheron 5 5 Pura raza española 17 10 21 48 Puro Sangre Lusitano 11 3 10 24 Quarter Horse 1 1 2 Selle Francais 1 1 Shetland Pony 15 2 22 39 RIVIA Memoria ejercicio 2020 Troteur Francais 1 1 Welsh 9 6 9 24 Asno 51 47 38 136 Andaluza 1 1 2 -

AKC Education Webinar Series: FSS/Miscellaneous Questions and Answers
AKC Education Webinar Series: FSS/Miscellaneous Questions and Answers 1. Can an FSS Parent Club offer an ATT Test? If the Parent Club is licensed to hold any event, it may offer an ATT Test. 2. For a domestically evolving breed that started with an independent registry for 20 years, then accepted into UKC for an additional 20 years, can these two be combined to qualify for the 40 years required to be considered into FSS, or must the 40 be done within the same registry body? The history of a breed having a registry for a minimum of 40 years can be merged as described, staff will review to determine if it would meet the parameters required. 3. Does AKC have reciprocity with UKC? AKC has open registration with individual breeds with that are registered by UKC. A breed requesting to be enrolled in the AKC FSS based upon UKC recognition would have reciprocity, if it meets the number of years being in existence, until the breed becomes AKC recognized. At which time the individual breeds may request to keep the studbook open for UKC registered dogs. 4. Is it a good practice to submit all of the required data to AKC in one PDF electronically to FSS as a final submission, once all criteria is met for the ease of processing? The FSS Department keeps track of required data, meeting minutes maybe a PDF, the other requirements need to be in different formats. Breed Standard needs to be a word document, membership list needs to be in an Excel as provided. -

Contact Details Number of Breeds Within FCI Groups List of Breeds
Contact details Last and First name of Judge Szczepańska-Korpetta Joanna Address 08-443 Sobienie Jeziory, Sobienie Kiełczewskie II - 17, Poland E-mail [email protected] Mobile (+48) 600 451 217 Home phone, Fax (+48) 22 839 65 84 ZKwP Branch Warszawa Languages Polish, German Number of breeds within FCI Groups FCI Group FCI group name Level 1 Sheepdogs and Cattle Dogs (except Swiss Cattle Dogs) (1/52) National 2 Pinscher and Schnauzer - Molossoid breeds - Swiss Mountain and Cattle Dogs International and other breeds (54/54) 3 Terriers (34/34) International 5 Spitz and primitive types (61/61) International 8 Retrievers - Flushing Dogs - Water Dogs (22/22) International 9 Companion and Toy Dogs (33/33) International List of breeds FCI Group Breed Level Sheepdogs and Cattle Dogs (except Swiss Cattle Dogs) 1 Polish Lowland Sheepdog (FCI - 251) National Pinscher and Schnauzer - Molossoid breeds - Swiss Mountain and Cattle Dogs and other breeds 2 Affenpinscher (FCI - 186) International 2 Alentejo Mastiff (FCI - 96) International 2 Appenzell Cattle Dog (FCI - 46) International 2 Atlas Mountain Dog - Aidi (FCI - 247) International 2 Austrian Pinscher (FCI - 64) International 2 Bernese Mountain Dog (FCI - 45) International 2 Bosnian and Herzegovinian - Croatian Shepherd Dog (FCI - 355) International 2 Boxer (FCI - 144) International 2 Broholmer (FCI - 315) International 2 Bulldog (FCI - 149) International 2 Bullmastiff (FCI - 157) International 2 Castro Laboreiro Dog (FCI - 170) International 2 Caucasian Shepherd Dog (FCI - 328) International 1 FCI Group -

Optivizor Sizing Manual V1,4
OPTIVIZOR EYE AND FACIAL PROTECTION - SIZING - v1.4 SIZING YOUR PET’S OPTIVIZOR The Optivizor is sized by head measurement and by weight using the tables provided. You may find that you have two different sizes according to the two tables. This is because, dogs come in all shapes and sizes even within the breed. When this happens, it is always better to size down. STEP 1 - ESTABLISH IF YOUR PET IS A NORMAL, SHORT OR LONG SNOUT BREED. STEP 2 - ESTABLISH THE WEIGHT A SNUG FIT ! STEP 3 - ESTABLISH THE HEAD MEASUREMENT When fitting your new Optivizor make sure the neck collar is done up STEP 4 - DETERMINE SIZE TO ORDER firmly - otherwise the dog is less likely to accept the collar quickly. Depending on the temperament of your dog, you may loosen this slightly once the Optivizor has been accepted. SIZE MATTERS ! A good fit is critical for the Optivizor to function correctly and to give your dog the best chance of accepting it. v1.4 STEP 1 - ESTABLISH IF YOUR PET IS A NORMAL, SHORT OR LONG SNOUT BREED. This is only a guideline due to possibility of variations within the breed itself. Dog breeds using normal snout Optivizor Affenpinscher German Short-/Long/ Munsterlander Airedale Terrier Wiredhaired Newfoundland Akita Inu Pointer Owtscharka American Collie Great Dane Pointer Appenzell Mountain Dog German Pinscher Podenco Australian Shepherd Foxhound Poodle Basset Hound Fox Terrier Komondor Bearded Collie Golden Retriever Rehpinscher Beagle Gordon Setter Rhodesian Ridgeback Bernese Mountain Dog Groenendael / Tervueren Rottweiler St. Bernhard German -
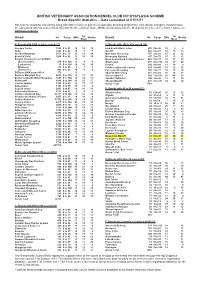
Breed Specific Stats BVA-KC Hip Scores
BRITISH VETERINARY ASSOCIATION/KENNEL CLUB HIP DYSPLASIA SCHEME Breed Specific Statistics – Data calculated to 01/11/11 Hip Scoring should be considered along with other criteria as part of a responsible breeding programme, and, ideally, breeders should choose breeding stock with hip scores WELL BELOW the Breed Mean Score (BMS) and ideally below the Median for their breed. For further advice see www.bva.co.uk/chs 5yr 5yr Breed No Range BMS Median Breed No Range BMS Median Mean Mean A: Breeds with 1000 or more scored (31) C: Breeds with 100 to 499 scored (29) Airedale Terrier 1560 0 to 91 16 14 12 Irish Red & White Setter 403 0 to 96 10 98 Akita 2342 0 to 92 10 77 Mastiff 366 0 to 81 18 19 13 Alaskan Malamute 1078 0 to 78 13 13 10 Maremma Sheepdog 157 2 to 83 15 23 12 Bearded Collie 3208 0 to 92 11 10 10 Norwegian Buhund 147 2 to 76 15 12 12 Belgian Shepherd (overall BMS) 10 Nova Scotia Duck Tolling Retriever 428 0 to 70 12 11 10 (Groenendael) 470 0 to 104 11 99 Otterhound 215 4 to 106 44 49 40 (Laekenois) 15 0 to 104 10 10 10 Pointer 142 0 to 60 11 10 9 (Malinois) 175 0 to 60 98 8 Polish Lowland Sheepdog 414 5 to 60 16 12 12 (Tervueren) 956 0 to 93 10 8 9 Pyrenean Sheepdog 105 3 to 77 14 13 12 BSD pre-2000 unspecified 179 12 Spanish Water Dog 217 0 to 64 16 16 12 Bernese Mountain Dog 4818 0 to 102 15 12 10 Sussex Spaniel 160 7 to 101 38 44 33 Border Collie/Working Sheepdog 7648 0 to 102 13 12 11 Swedish Vallhund 190 2 to 40 11 11 10 Bullmastiff 1049 0 to 104 26 22 17 Tibetan Mastiff 225 0 to 101 14 14 10 Cocker Spaniel 1100 0 to 99 13 -

The Bernese Is a Loyal Dog of the Swiss Alps
The Bernese Is A Loyal Dog Of The Swiss Alps By MRS. L. EGG LEACH Questa vom Sumiswald the small wagons, filled with baskets, is an excellent specimen to the market place, in Berne. of the Bernese Mountain Dog. She is exceedingly fond of EVERTHELESS, they were Children, as are all mem- passed by unnoticed for many bers of the breed years. Their brave deeds were never brought to the notice of the public, at home or abroad. Still these dogs did their duty, year in and year out, often under the greatest hardships, and without the kindly and loving care that are bestowed on the St. Bernards by the monks at the Hospice. HERE are four species of Swiss but it is the one with which I am most Their masters—the basket weavers — mountain dogs, • or familiar, having always had the good were reared in a hard school. Work and "Sennenhunde," as they are fortune to have some in my near neigh- poverty was often their lot. They lived called in Switzerland. They are: the borhood, which I could watch and miles from anywhere, in some lonely Appenzeller Sennenhunde from the study individually. mountain valley or hamlet, and their Canton of Appenzell, the smallest of I am not a fancier of this breed, but I only recreation was the long tramp, the four species; the Entlibucher have watched it closely, without taking beside their wagons, on market-day. Sennenhunde, from Entlibuch in the any active part, for very many years, Canton of Lucerne; the Grosse and my admiration has never waned. -

Inbreeding and Relationship in the German Shepherd Dog Population in Area of Cracow Branch of Polish Kennel Club
Scientific Annals of Polish Society of Animal Production - Vol. 7 (2011), No 3, 21-29 Inbreeding and relationship in the German Shepherd dog population in area of Cracow Branch of Polish Kennel Club Joanna Kania-Gierdziewicz1, Bożena Kalinowska2, Maciej Gierdziewicz1 1University of Agriculture in Cracow, Faculty of Animal Sciences, Department of Genetics and Animal Breeding, al. Mickiewicza 24/28, 30-059 Kraków, e-mail: [email protected] 2Cracow Branch of the Polish Kennel Club, ul. Żywiecka 36, 30-427 Kraków The German Shepherd seems to be most popular and widely used dog breed in the world and also in Poland. The aim of the work was to estimate inbreeding and relationship coefficients in the population of German Shepherd dogs from the herdbook of Cracow Branch of the Polish Kennel Club. As the material, four-generation pedigrees of 60 animals (17 dogs and 43 bitches) born in 1994-2005 were used. The inbreeding (FX) and relationship (RXY) coefficients among all animals for each sex separately and RXY between dogs and bitches were calculated. Among all 60 animals 54 were inbred (90%). The FX values for all and for inbred animals were: 0.1286 and 0.1429, respectively. In the group of 17 males 16 were inbred (over 94%) and in the group of 43 females 38 were inbred (88.4%). Average FX was 0.1560, 0.1178, 0.1658, 0.1333 for all dogs, all bitches, inbred dogs and inbred bitches, respectively. About 98.9% of 1770 pairs of individuals were related; for all and related pairs the values of RXY were 0.0391 and 0.0395, respectively. -

Breeds of Cats and Dogs
Breeds of Dogs and Cats Floron C. Faries, Jr. DVM, MS Objectives Discuss the evolution of man’s relationship with dogs and cats Describe the characteristics shared by members of the Canidae family Describe the classification system for dogs List the uses for different breeds of dogs Identify and describe the different breeds of dogs Describe the characteristics shared by members of the family Felidae Describe the classification system for cats Identify and describe the different cat breeds History of Dogs In family Canidae Direct descendents of the wolf Wolf’s scientific name – Canis lupus Dog’s scientific name – Canis familiaris Domestication a few 1,000 years Greece Herding dogs Guarding dogs Hunting dogs Egypt Dogs used in war Bred based on purpose Climate Environment Master’s preference – herding, guarding, hunting 72 million dogs live in U.S. One dog per household in half American family homes More than 228 pure breeds More than 100 mixed breeds Stimulate income of dog industries $11 billion annual sales of dog food Accessory manufacturers Veterinarians Pharmaceutical industry Breeders Racers Trainers Herders Hunters Serve humans Protection Sight Hearing Security Companionship Characteristics of Dogs Size Height 6 inches to 40 inches at the shoulder Life expectancy 9 to 15 years, some 20 years Small dogs live longer than large dogs Common traits Shed hair once a year Non-retractable claws 42 adult teeth Pointed canine teeth Sweating Sweat glands on nose and feet Panting Hearing