Status of Scientific Knowledge, Recovery Progress, and Future Research Directions for the Gulf Sturgeon, Acipenser Oxyrinchus Desotoi Vladykov, 1955 K.J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fish Consumption Guidelines: Rivers & Creeks
FRESHWATER FISH CONSUMPTION GUIDELINES: RIVERS & CREEKS NO RESTRICTIONS ONE MEAL PER WEEK ONE MEAL PER MONTH DO NOT EAT NO DATA Bass, LargemouthBass, Other Bass, Shoal Bass, Spotted Bass, Striped Bass, White Bass, Bluegill Bowfin Buffalo Bullhead Carp Catfish, Blue Catfish, Channel Catfish,Flathead Catfish, White Crappie StripedMullet, Perch, Yellow Chain Pickerel, Redbreast Redhorse Redear Sucker Green Sunfish, Sunfish, Other Brown Trout, Rainbow Trout, Alapaha River Alapahoochee River Allatoona Crk. (Cobb Co.) Altamaha River Altamaha River (below US Route 25) Apalachee River Beaver Crk. (Taylor Co.) Brier Crk. (Burke Co.) Canoochee River (Hwy 192 to Ogeechee River) Chattahoochee River (Helen to Lk. Lanier) (Buford Dam to Morgan Falls Dam) (Morgan Falls Dam to Peachtree Crk.) * (Peachtree Crk. to Pea Crk.) * (Pea Crk. to West Point Lk., below Franklin) * (West Point dam to I-85) (Oliver Dam to Upatoi Crk.) Chattooga River (NE Georgia, Rabun County) Chestatee River (below Tesnatee Riv.) Conasauga River (below Stateline) Coosa River (River Mile Zero to Hwy 100, Floyd Co.) Coosa River <32" (Hwy 100 to Stateline, Floyd Co.) >32" Coosa River (Coosa, Etowah below Thompson-Weinman dam, Oostanaula) Coosawattee River (below Carters) Etowah River (Dawson Co.) Etowah River (above Lake Allatoona) Etowah River (below Lake Allatoona dam) Flint River (Spalding/Fayette Cos.) Flint River (Meriwether/Upson/Pike Cos.) Flint River (Taylor Co.) Flint River (Macon/Dooly/Worth/Lee Cos.) <16" Flint River (Dougherty/Baker Mitchell Cos.) 16–30" >30" Gum Crk. (Crisp Co.) Holly Crk. (Murray Co.) Ichawaynochaway Crk. Kinchafoonee Crk. (above Albany) Little River (above Clarks Hill Lake) Little River (above Ga. Hwy 133, Valdosta) Mill Crk. -
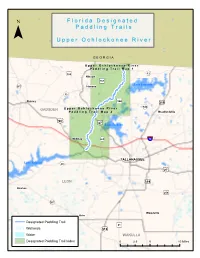
Upper Ochlockonee River Paddling Guide
F ll o r ii d a D e s ii g n a tt e d ¯ P a d d ll ii n g T r a ii ll s U p p e r O c h ll o c k o n e e R ii v e r G E O R G I A U p p e rr O c h ll o c k o n e e R ii v e rr P a d d ll ii n g T rr a ii ll M a p 1 159 «¬12 )" Hinson )"157 343 Lake Iamonia )" «¬267 Havana «¬12 344 Quincy )" ¤£319 342 GADSDEN U p p e rr O c h ll o c k o n e e R ii v e rr «¬ P a d d ll ii n g T rr a ii ll M a p 2 Bradfordville 90 ¤£ 27 ¤£ Lake Jackson Midway «¬263 ¨¦§10 1)"541 Capitola TALLAHASSEE Lake Talquin «¬20 «¬267 ¤£27 LEON ¤£319 Bloxham )"259 «¬267 Woodville Helen Designated Paddling Trail )"61 Wetlands ¤£319 Water WAKULLA Designated Paddling Trail Index 0 2.5 5 10 Miles 319 ¤£ 61 Newport Arran )" U p p e rr O c h ll o c k o n e e R ii v e rr P a d d ll ii n g T rr a ii ll M a p 1 ¯ Bell Rd d R d or n c Co o ir a C !| River Ridge «¬12 Plantation Concord Conservation Easement Access Point 1: SR 12 N: 30.6689 W: -84.3051 Havana Hiamonee à Plantation Conservation «¬12 Easement River Ridge Plantation C Conservation o n c Easement o d r R d n a R i id d r e M Kemp Rd N E D Tall Timbers Research Station S D & Land Conservancy A N O G E L Lake Iamonia I ro n B r id g e R d d Pond R ard ch Or !| Mallard Pond Access Point 2: Old Bainbridge Rd Bridge N: 30.5858 W: -84.3594 O l d B Carr Lake a i n b r i d Upper Ochlockonee River Paddling Trail g e R Canoe/Kayak Launch d !| Conservation Lands 27 0 0.5 1 2 Miles ¤£ Wetlands ¯ U p p e rr O c h ll o c k o n e e R ii v e rr P a d d ll ii n g T rr a ii ll M a p 2 )"270 RCM Farms Conservation Easement O l d B a i -

Pan-European Action Plan for Sturgeons
Strasbourg, 30 November 2018 T-PVS/Inf(2018)6 [Inf06e_2018.docx] CONVENTION ON THE CONSERVATION OF EUROPEAN WILDLIFE AND NATURAL HABITATS Standing Committee 38th meeting Strasbourg, 27-30 November 2018 PAN-EUROPEAN ACTION PLAN FOR STURGEONS Document prepared by the World Sturgeon Conservation Society and WWF This document will not be distributed at the meeting. Please bring this copy. Ce document ne sera plus distribué en réunion. Prière de vous munir de cet exemplaire. T-PVS/Inf(2018)6 - 2 - Pan-European Action Plan for Sturgeons Multi Species Action Plan for the: Russian sturgeon complex (Acipenser gueldenstaedtii, A. persicus-colchicus), Adriatic sturgeon (Acipenser naccarii), Ship sturgeon (Acipenser nudiventris), Atlantic/Baltic sturgeon, (Acipenser oxyrinchus), Sterlet (Acipenser ruthenus), Stellate sturgeon (Acipenser stellatus), European/Common sturgeon (Acipenser sturio), and Beluga (Huso huso). Geographical Scope: European Union and neighbouring countries with shared basins such as the Black Sea, Mediterranean, North Eastern Atlantic Ocean, North Sea and Baltic Sea Intended Lifespan of Plan: 2019 – 2029 Russian Sturgeon Adriatic Sturgeon Ship Sturgeon Atlantic or Baltic complex Sturgeon Sterlet Stellate Sturgeon Beluga European/Common Sturgeon © M. Roggo f. A. sturio; © Thomas Friedrich for all others Supported by - 3 - T-PVS/Inf(2018)6 GEOGRAPHICAL SCOPE: The Action Plan in general addresses the entire Bern Convention scope (51 Contracting Parties, including the European Union) and in particular the countries with shared sturgeon waters in Europe. As such, it focuses primarily on the sea basins in Europe: Black Sea, Mediterranean, North-East Atlantic, North Sea, Baltic Sea, and the main rivers with relevant current or historic sturgeon populations (see Table 2). -

Final NASPS Newsletter V2I2 Cover
North American Sturgeon and Paddlefish Society THE LIVING FOSSIL Volume 2 ISSUE 2 | SEPTEMBER 2015 IN THIS ISSUE Society updates MEETING DETAILS Student travel awards AWARD WINNERS In the news FISHING, RESEARCH, AND REGULATIONS Scaphirhynchus spotlight SHOVELNOSE STURGEON 70+ years of managing a successful Lake Sturgeon fishery WINNEBAGO SYSTEM OSHKOSH 2015! LAKE STURGEON 72 PRESENTATIONS Feature Article: Highlighting sturgeon and 19 POSTERS paddlefish recovery teams 3 WORKSHOPS SOUNDS SHED LIGHT ON 4 FORUM DISCUSSIONS GULF STURGEON SOCIALS, BANQUET & MORE! AND THEIR HABITATS NASPS MISSION STATEMENT The North American Sturgeon and Paddlefish Society (NASPS) was founded in 2012 at the Annual Meeting of the American Fisheries Society in St. Paul, MN, and is the North American affiliate of the World Sturgeon Conservation Society. Seeking to address current declines in sturgeon and paddlefish populations across North America, NASPS is dedicated to promoting the conservation and restoration of these species by developing and advancing research pertaining to their biology, management, and utilization. Distributing scientific, cultural, and historical information related to sturgeon and paddlefish in North America and encouraging the inclusion of sturgeon and paddlefish fisheries science in college and continuing education curricula is critical to achieving these objectives. As such, NASPS recognizes the importance of engaging NASPS members, local, national, and international government organizations, educational institutions, non-governmental -
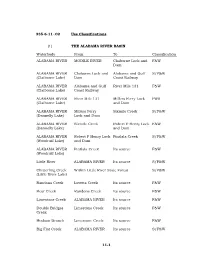
11-1 335-6-11-.02 Use Classifications. (1) the ALABAMA RIVER BASIN Waterbody from to Classification ALABAMA RIVER MOBILE RIVER C
335-6-11-.02 Use Classifications. (1) THE ALABAMA RIVER BASIN Waterbody From To Classification ALABAMA RIVER MOBILE RIVER Claiborne Lock and F&W Dam ALABAMA RIVER Claiborne Lock and Alabama and Gulf S/F&W (Claiborne Lake) Dam Coast Railway ALABAMA RIVER Alabama and Gulf River Mile 131 F&W (Claiborne Lake) Coast Railway ALABAMA RIVER River Mile 131 Millers Ferry Lock PWS (Claiborne Lake) and Dam ALABAMA RIVER Millers Ferry Sixmile Creek S/F&W (Dannelly Lake) Lock and Dam ALABAMA RIVER Sixmile Creek Robert F Henry Lock F&W (Dannelly Lake) and Dam ALABAMA RIVER Robert F Henry Lock Pintlala Creek S/F&W (Woodruff Lake) and Dam ALABAMA RIVER Pintlala Creek Its source F&W (Woodruff Lake) Little River ALABAMA RIVER Its source S/F&W Chitterling Creek Within Little River State Forest S/F&W (Little River Lake) Randons Creek Lovetts Creek Its source F&W Bear Creek Randons Creek Its source F&W Limestone Creek ALABAMA RIVER Its source F&W Double Bridges Limestone Creek Its source F&W Creek Hudson Branch Limestone Creek Its source F&W Big Flat Creek ALABAMA RIVER Its source S/F&W 11-1 Waterbody From To Classification Pursley Creek Claiborne Lake Its source F&W Beaver Creek ALABAMA RIVER Extent of reservoir F&W (Claiborne Lake) Beaver Creek Claiborne Lake Its source F&W Cub Creek Beaver Creek Its source F&W Turkey Creek Beaver Creek Its source F&W Rockwest Creek Claiborne Lake Its source F&W Pine Barren Creek Dannelly Lake Its source S/F&W Chilatchee Creek Dannelly Lake Its source S/F&W Bogue Chitto Creek Dannelly Lake Its source F&W Sand Creek Bogue -

City of Jacksonville/Duval County Environmental Review Record CDBG-DR – Hurricane Matthew Housing Repair Program Contents Scope of Work
City of Jacksonville/Duval County Environmental Review Record CDBG-DR – Hurricane Matthew Housing Repair Program Contents Scope of Work ................................................................................................................................ 1 Broad-Level Tiered Environmental Review ................................................................................... 3 Supporting Documentation ....................................................................................................... 11 Unmet Needs ......................................................................................................................... 11 Project Area ........................................................................................................................... 12 Airport Hazards ..................................................................................................................... 13 Coastal Barrier Resources ..................................................................................................... 17 Flood Insurance ..................................................................................................................... 19 Clean Air................................................................................................................................ 22 Coastal Zone Management .................................................................................................... 26 Endangered Species .............................................................................................................. -

Chapter 335-6-11 Water Use Classifications for Interstate and Intrastate Waters
Environmental Management Chapter 335-6-11 DEPARTMENT OF ENVIRONMENTAL MANAGEMENT WATER DIVISION - WATER QUALITY PROGRAM ADMINISTRATIVE CODE CHAPTER 335-6-11 WATER USE CLASSIFICATIONS FOR INTERSTATE AND INTRASTATE WATERS TABLE OF CONTENTS 335-6-11-.01 The Use Classification System 335-6-11-.02 Use Classifications 335-6-11-.01 The Use Classification System. (1) Use classifications utilized by the State of Alabama are as follows: Outstanding Alabama Water ................... OAW Public Water Supply ......................... PWS Swimming and Other Whole Body Shellfish Harvesting ........................ SH Fish and Wildlife ........................... F&W Limited Warmwater Fishery ................... LWF Agricultural and Industrial Water Supply ................................ A&I (2) Use classifications apply water quality criteria adopted for particular uses based on existing utilization, uses reasonably expected in the future, and those uses not now possible because of correctable pollution but which could be made if the effects of pollution were controlled or eliminated. Of necessity, the assignment of use classifications must take into consideration the physical capability of waters to meet certain uses. (3) Those use classifications presently included in the standards are reviewed informally by the Department's staff as the need arises, and the entire standards package, to include the use classifications, receives a formal review at least once every three years. Efforts currently underway through local 201 planning projects will provide additional technical data on certain waterbodies in the State, information on treatment alternatives, and applicability of various management techniques, which, when available, will hopefully lead to new decisions regarding use classifications. Of particular interest are those segments which are currently classified for any usage which has an associated Supp. -

Template for Hazard Mitigation Plan
HAZARD MITIGATION PLAN FOR COVINGTON COUNTY, ALABAMA (2009 UPDATE DRAFT) Covington County Hazard Mitigation Plan Table of Contents Section 1 Hazard Mitigation Plan Background 1.1 Introduction 1.2 Authority 1.3 Funding 1.4 Scope 1.5 Purpose Section 2 Covington County Profile 2.1 Geology 2.2 Transportation 2.3 Economy 2.4 Utilities 2.5 Social and Economic Characteristics Section 3 Planning Process 3.1 Multi-Jurisdictional Plan Adoption 3.2 Multi-Jurisdictional Planning Participation 3.3 Hazard Mitigation Planning Process 3.4 Public and Other Stakeholder Involvement 3.5 Integration with Existing Plans Section 4 Risk Assessment 4.1 Hazard Identification 4.2 Susceptibility to Hazards by Jurisdiction 4.3 Extent of Hazards by Jurisdiction 4.4 Previous Occurrences 4.5 Past Occurrence Documentation 4.6 Probability of Future Occurrence by Jurisdiction 4.7 Vulnerability Overview 4.8 Population Vulnerable to Hazards by Jurisdiction 4.9 Socially Vulnerable Populations 4.10 County Building Stock 4.11 Critical Facilities/Infrastructure Identification 4.12 Critical Facilities/Infrastructure Identification by Jurisdiction 4.13 Repetitive Loss Properties 4.14 Analyzing Development Trends Section 5 Mitigation 5.1 Mitigation Planning Process 5.2 Mitigation Goals and Strategies by Jurisdiction Covington County City of Andalusia Town of Babbie Town of Carolina City of Florala Town of Gantt Town of Heath 2 Covington County Hazard Mitigation Plan Town of Horn Hill Town of Libertyville Town of Lockhart Town of Onycha City of Opp Town of Red Level Town of River -

Atlantic Sturgeon Acipenser Oxyrinchus
COSEWIC Assessment and Status Report on the Atlantic Sturgeon Acipenser oxyrinchus St. Lawrence populations Maritimes populations in Canada THREATENED 2011 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC. 2011. COSEWIC assessment and status report on the Atlantic Sturgeon Acipenser oxyrinchus in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xiii + 49 pp. (www.sararegistry.gc.ca/status/status_e.cfm). Production note: COSEWIC acknowledges Robert Campbell for writing the provisional status report on the Atlantic Sturgeon, Acipenser oxyrinchus. The contractor’s involvement with the writing of the status report ended with the acceptance of the provisional report. Any modifications to the status report during the subsequent preparation of the 6-month interim and 2-month interim status report were overseen by Dr. Eric Taylor, COSEWIC Freshwater Fishes Specialist Subcommittee Co-Chair. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: 819-953-3215 Fax: 819-994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur l'esturgeon noir (Acipenser oxyrinchus) au Canada. Cover illustration/photo: Atlantic Sturgeon — from Cornell University Department of Natural Resources by permission. Her Majesty the Queen in Right of Canada, 2011. Catalogue No. CW69-14/636-2011E-PDF ISBN 978-1-100-18706-8 Recycled paper COSEWIC Assessment Summary Assessment Summary – May 2011 Common name Atlantic Sturgeon - St. -

And Type the TITLE of YOUR WORK in All Caps
POTENTIAL THREATS OF THE EXOTIC APPLE SNAIL POMACEA INSULARUM TO AQUATIC ECOSYSTEMS IN GEORGIA AND FLORIDA by SHELLEY MARIE ROBERTSON (Under the Direction of Susan Bennett Wilde) ABSTRACT The Island apple snail, Pomacea insularum is a freshwater gastropod in the family Ampullaridae. It was introduced into the United States via the aquarium trade approximately 30 years ago and now has established reproducing populations in at least seven southeastern states. It is a highly invasive species with high rates of reproduction and consumption of native aquatic vegetation. A survey of reported Georgia populations confirmed that there are at least ten individual occurrences of exotic apple snails in the state, and that they have not reached their equilibrium distribution. We also investigated the ability of P. insularum to harbor a cyanotoxin that may be detrimental to its avian predator in Florida, the endangered Florida snail kite (Rostrhamus sociabilis). The invasive P. insularum transferred the undescribed cyanotoxin associated with Avian Vacuolar Myelinopathy to domestic chickens in a laboratory feeding study. INDEX WORDS: apple snail, Pomacea insularum, invasive species, Avian Vacuolar Myelinopathy, AVM, Florida snail kite, Rostrhamus sociabilis, Hydrilla verticillata POTENTIAL THREATS OF THE EXOTIC APPLE SNAIL POMACEA INSULARUM TO AQUATIC ECOSYSTEMS IN GEORGIA AND FLORIDA by SHELLEY MARIE ROBERTSON BS, University of Georgia, 2006 AB, University of Georgia, 2007 A Thesis Submitted to the Graduate Faculty of The University of Georgia in Partial Fulfillment of the Requirements for the Degree MASTER OF SCIENCE ATHENS, GEORGIA 2012 © 2012 Shelley Marie Robertson All Rights Reserved POTENTIAL THREATS OF THE EXOTIC APPLE SNAIL POMACEA INSULARUM TO AQUATIC ECOSYSTEMS IN GEORGIA AND FLORIDA by SHELLEY MARIE ROBERTSON Major Professor: Susan B. -

Acipenser Oxyrinchus) Ecological Risk Screening Summary
Atlantic sturgeon (Acipenser oxyrinchus) Ecological Risk Screening Summary U.S. Fish and Wildlife Service, web version – 03/29/2018 Photo: Simon Pierre Barrette. Licensed under CC BY-SA 3.0. Available: https://commons.wikimedia.org/wiki/File:Acipenser_oxyrinchus_PAQ.jpg. 1 Native Range and Status in the United States Native Range From Froese and Pauly (2014): “Western Atlantic: Hamilton River, Labrador, Newfoundland, Canada to northeastern Florida, USA. Occurs occasionally in Bermuda and French Guiana [Robins and Ray 1986]. Northern Gulf of Mexico [Smith 1997]. In Europe: Baltic Sea. Landlocked populations in Lakes Ladoga and Onega (Russia), both now extirpated. Occasionally recorded from Great Britain and North Sea in Elbe drainage [Kottelat and Freyhof 2007]. Recent research revealed that this species existed in the Baltic Sea, but is now extirpated [Ludwig et al. 2002, 2008].” 1 Status in the United States From Froese and Pauly (2014): “USA: native” From NOAA Fisheries (2016): “Historically, Atlantic sturgeon were present in approximately 38 rivers in the United States from St. Croix, ME to the Saint Johns River, FL, of which 35 rivers have been confirmed to have had a historical spawning population. Atlantic sturgeon are currently present in approximately 32 of these rivers, and spawning occurs in at least 20 of them.” GBIF Secretariat (2014) contains a record in California that is assumed to be a misidentification as it is from 1956 and not substantiated elsewhere. Means of Introductions in the United States No records of non-native Acipenser oxyrinchus introductions in the United States were found. Remarks From Froese and Pauly (2014): “Near threatened globally, but extirpated in Europe [Kottelat and Freyhof 2007]. -

Suwannee River State Park
HISTORY AND NATURE SUWANNEE RIVER The park contains over 1,800 acres of natural STATE PARK Florida with many features such as sinks, streams, 3631 201st Path SUWANNEE RIVER springs, limestone outcroppings and the rivers. Live Oak, FL 32060 STATE PARK The park has an abundance of plant and animal 386-362-2746 species including gopher tortoise, fox, deer, song Where the scenic Withlacoochee birds, wildflowers and diverse native forests. The joins the historic Suwannee protected Gulf Sturgeon and other fishes and reptiles are abundant in the river. PARK GUIDELINES Early use by Native Americans dates back some • Hours are 8 a.m. until sunset, 365 days a year. 12,000 years. While under Spanish control, the • An entrance fee is required. passage of De Soto’s party occurred in 1540. During • All plants, animals and park property are 1818 Andrew Jackson lead American forces through protected. Collection, destruction or disturbance this area searching for Indian strongholds, believed is prohibited. responsible for raiding settlers. • Pets are permitted in designated areas only. Pets Vestiges of history in the park show how important must be kept on a handheld leash no longer the Suwannee River was to Florida history. One can than six feet and well-behaved at all times. find an earthworks mound built during the Civil • Fishing, boating and fires are allowed in War to defend the railroad crossing that supplied designated areas only. A Florida fishing license confederate troops. The Battle of Olustee in may be required. February 1864 turned back Union forces heading west to destroy this bridge.