And Type the TITLE of YOUR WORK in All Caps
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cipangopaludina Chinensis: Effects of Temperatures and Parasite Prevalence
Cipangopaludina chinensis: Effects of temperatures and parasite prevalence BIOS 35502-01: Practicum in Environmental Field Biology Jennifer Lam Advisor: Sara Benevente 2016 Lam 2 Abstract Comparison of parasite prevalence between native snail species, Lymnaea stagnalis, and invasive snail species, Cipangopaludina chinensis, was conducted by euthanizing snails one day after capture from Brown Lake. Looking at if parasitism is a main factor in intraspecific competition of C. chinensis at different temperatures, snails were captured, measured for height and width, housed under different temperature settings (20◦C, 25◦C, and 34◦C), and competed against other for a week-long trial. After the week-long trial or when the last snail died, snails were re-measured for growth and checked for parasites. Result from parasite prevalence between two snails species was significant, C. chinensis rarely had infections compared to L. stagnalis. From the result, native parasites were predicted to be highly specialized for L. stagnalis and lack mechanisms to penetrate C. chinensis. Result from the intraspecific competition suggested that parasite prevalence was not important as survival (in weeks) in the change in growth. Since parasite prevalence was low, and sample size was small, conclusion on parasite prevalence not being a factor in growth was not robust. Further studies on impact of C. chinensis on freshwater systems and native snail species are needed. Introduction Climate change is a concerning factor for freshwater ecosystems. Climate change increases the average temperature of water in some aquatic systems. With warmer water temperatures, more exotic species can establish as native species disappear (Rahel and Olden 2008). As native species disappear from the ecosystem, other species dependent on a specific native species may not be able to sustain the loss, leading to a decrease in biodiversity. -

Fish Consumption Guidelines: Rivers & Creeks
FRESHWATER FISH CONSUMPTION GUIDELINES: RIVERS & CREEKS NO RESTRICTIONS ONE MEAL PER WEEK ONE MEAL PER MONTH DO NOT EAT NO DATA Bass, LargemouthBass, Other Bass, Shoal Bass, Spotted Bass, Striped Bass, White Bass, Bluegill Bowfin Buffalo Bullhead Carp Catfish, Blue Catfish, Channel Catfish,Flathead Catfish, White Crappie StripedMullet, Perch, Yellow Chain Pickerel, Redbreast Redhorse Redear Sucker Green Sunfish, Sunfish, Other Brown Trout, Rainbow Trout, Alapaha River Alapahoochee River Allatoona Crk. (Cobb Co.) Altamaha River Altamaha River (below US Route 25) Apalachee River Beaver Crk. (Taylor Co.) Brier Crk. (Burke Co.) Canoochee River (Hwy 192 to Ogeechee River) Chattahoochee River (Helen to Lk. Lanier) (Buford Dam to Morgan Falls Dam) (Morgan Falls Dam to Peachtree Crk.) * (Peachtree Crk. to Pea Crk.) * (Pea Crk. to West Point Lk., below Franklin) * (West Point dam to I-85) (Oliver Dam to Upatoi Crk.) Chattooga River (NE Georgia, Rabun County) Chestatee River (below Tesnatee Riv.) Conasauga River (below Stateline) Coosa River (River Mile Zero to Hwy 100, Floyd Co.) Coosa River <32" (Hwy 100 to Stateline, Floyd Co.) >32" Coosa River (Coosa, Etowah below Thompson-Weinman dam, Oostanaula) Coosawattee River (below Carters) Etowah River (Dawson Co.) Etowah River (above Lake Allatoona) Etowah River (below Lake Allatoona dam) Flint River (Spalding/Fayette Cos.) Flint River (Meriwether/Upson/Pike Cos.) Flint River (Taylor Co.) Flint River (Macon/Dooly/Worth/Lee Cos.) <16" Flint River (Dougherty/Baker Mitchell Cos.) 16–30" >30" Gum Crk. (Crisp Co.) Holly Crk. (Murray Co.) Ichawaynochaway Crk. Kinchafoonee Crk. (above Albany) Little River (above Clarks Hill Lake) Little River (above Ga. Hwy 133, Valdosta) Mill Crk. -

First Report of the Invasive Snail Pomacea Canaliculata in Kenya Alan G
Buddie et al. CABI Agric Biosci (2021) 2:11 https://doi.org/10.1186/s43170-021-00032-z CABI Agriculture and Bioscience RESEARCH Open Access First report of the invasive snail Pomacea canaliculata in Kenya Alan G. Buddie1* , Ivan Rwomushana2 , Lisa C. Oford1 , Simeon Kibet3, Fernadis Makale2 , Djamila Djeddour1 , Giovanni Cafa1 , Koskei K. Vincent4, Alexander M. Muvea3 , Duncan Chacha2 and Roger K. Day2 Abstract Following reports of an invasive snail causing crop damage in the expansive Mwea irrigation scheme in Kenya, samples of snails and associated egg masses were collected and sent to CABI laboratories in the UK for molecular identifcation. DNA barcoding analyses using the cytochrome oxidase subunit I gene gave preliminary identifcation of the snails as Pomacea canaliculata, widely considered to have the potential to be one of the most invasive inver- tebrates of waterways and irrigation systems worldwide and which is already causing issues throughout much of south-east Asia. To the best of our knowledge, this is the frst documented record of P. canaliculata in Kenya, and the frst confrmed record of an established population in continental Africa. This timely identifcation shows the beneft of molecular identifcation and the need for robust species identifcations: even a curated sequence database such as that provided by the Barcoding of Life Data system may require additional checks on the veracity of the underlying identifcations. We found that the egg mass tested gave an identical barcode sequence to the adult snails, allowing identifcations to be made more rapidly. Part of the nuclear elongation factor 1 alpha gene was sequenced to confrm that the snail was P. -
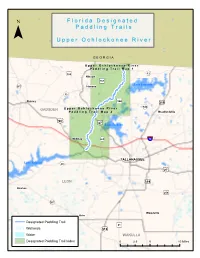
Upper Ochlockonee River Paddling Guide
F ll o r ii d a D e s ii g n a tt e d ¯ P a d d ll ii n g T r a ii ll s U p p e r O c h ll o c k o n e e R ii v e r G E O R G I A U p p e rr O c h ll o c k o n e e R ii v e rr P a d d ll ii n g T rr a ii ll M a p 1 159 «¬12 )" Hinson )"157 343 Lake Iamonia )" «¬267 Havana «¬12 344 Quincy )" ¤£319 342 GADSDEN U p p e rr O c h ll o c k o n e e R ii v e rr «¬ P a d d ll ii n g T rr a ii ll M a p 2 Bradfordville 90 ¤£ 27 ¤£ Lake Jackson Midway «¬263 ¨¦§10 1)"541 Capitola TALLAHASSEE Lake Talquin «¬20 «¬267 ¤£27 LEON ¤£319 Bloxham )"259 «¬267 Woodville Helen Designated Paddling Trail )"61 Wetlands ¤£319 Water WAKULLA Designated Paddling Trail Index 0 2.5 5 10 Miles 319 ¤£ 61 Newport Arran )" U p p e rr O c h ll o c k o n e e R ii v e rr P a d d ll ii n g T rr a ii ll M a p 1 ¯ Bell Rd d R d or n c Co o ir a C !| River Ridge «¬12 Plantation Concord Conservation Easement Access Point 1: SR 12 N: 30.6689 W: -84.3051 Havana Hiamonee à Plantation Conservation «¬12 Easement River Ridge Plantation C Conservation o n c Easement o d r R d n a R i id d r e M Kemp Rd N E D Tall Timbers Research Station S D & Land Conservancy A N O G E L Lake Iamonia I ro n B r id g e R d d Pond R ard ch Or !| Mallard Pond Access Point 2: Old Bainbridge Rd Bridge N: 30.5858 W: -84.3594 O l d B Carr Lake a i n b r i d Upper Ochlockonee River Paddling Trail g e R Canoe/Kayak Launch d !| Conservation Lands 27 0 0.5 1 2 Miles ¤£ Wetlands ¯ U p p e rr O c h ll o c k o n e e R ii v e rr P a d d ll ii n g T rr a ii ll M a p 2 )"270 RCM Farms Conservation Easement O l d B a i -

Population Dynamics of Pomacea Flagellata
Limnetica, 29 (2): x-xx (2011) Limnetica, 34 (1): 69-78 (2015). DOI: 10.23818/limn.34.06 c Asociación Ibérica de Limnología, Madrid. Spain. ISSN: 0213-8409 Population dynamics of the native apple snail Pomacea flagellata (Ampullariidae) in a coastal lagoon of the Mexican Caribbean Frank A. Ocaña∗, Alberto de Jesús-Navarrete, José Juan Oliva-Rivera, Rosa María de Jesús- Carrillo and Abel Abraham Vargas-Espósitos1 Departamento de Sistemática y Ecología Acuática. El Colegio de la Frontera Sur (ECOSUR), Unidad Chetumal. Av. Centenario km 5.5, Chetumal, Quintana Roo, México ∗ Corresponding author: [email protected] 2 Received: 25/07/2014 Accepted: 20/11/2014 ABSTRACT Population dynamics of the native apple snail Pomacea flagellata (Ampullariidae) in a coastal lagoon of the Mexican Caribbean Apple snails Pomacea spp inhabit tropical and subtropical freshwater environments and are of ecological and economic importance. To evaluate the population dynamics of P. flagellata, monthly samples were collected from Guerrero Lagoon (Yucatán Peninsula) from June 2012 to May 2013. The measured environmental variables did not differ significantly among the sampling stations. However, salinity was lower during the rainy season, and the temperature was lower during the north season (i.e., the season dominated by cold fronts). The snails were more abundant during the rainy season, and they were restricted to the portion of the lagoon that receives freshwater discharges. The snails ranged from 4 to 55 mm in size, with a –1 maximum estimated length of L∞ = 57.75 mm and a growth rate of K = 0.68 y (the abbreviation “y” means “year”) with a seasonal oscillation; the lowest growth rate occurred in early December. -

Applesnails of Florida Pomacea Spp. (Gastropoda: Ampullariidae) 1 Thomas R
EENY323 Applesnails of Florida Pomacea spp. (Gastropoda: Ampullariidae) 1 Thomas R. Fasulo2 Introduction in the northern tier of Florida counties and northward except where the water is artificially heated by industrial Applesnails are larger than most freshwater snails and can wastewater or in warm springs. It occurs as far west as be separated from other freshwater species by their oval the Choctawhatchee River. It is easily distinguished from shell that has the umbilicus (the axially aligned, hollow, other applesnails in Florida by the low, strongly rounded cone-shaped space within the whorls of a coiled mollusc shell spike, and measures about 40–70 mm (Capinera and shell) of the shell perforated or broadly open. There are four White 2011). species of Pomacea in Florida, one of which is native and considered beneficial (Capinera and White 2011). Species Found in Florida Of the four species of applesnails in Florida, only the Florida applesnail is a native species, while the other three species are introduced. All are tropical/subtropical species in the genus Pomacea, and are not known to withstand water temperatures below 10°C (FFWCC 2006). • Pomacea paludosa (Say 1829), the Florida applesnail, occurs throughout peninsular Florida (Thompson 1984). Based on fossil finds, it is a native snail that has existed in Florida since the Pliocene. It is also native to Cuba and Hispaniola (FFWCC 2006). Collections have been made in Alabama, Georgia, Hawaii, Louisiana, Oklahoma and South Carolina (USGS 2006). It is the principal Figure 1. Florida applesnail, Pomacea paludosa (Say 1829). food of the Everglades kite, Rostrhamus sociabilis Credits: Bill Frank, http://www.jacksonvilleshells.org plumbeus Ridgway, and should be considered beneficial. -

Pomacea Canaliculata (Lamarck, 1822)
Pomacea canaliculata (Lamarck, 1822) Diagnostic features Distinguished from Pomacea diffusa by its larger sized shell (up to 75 mm in height) and deeply channelled suture. Animal with distinctive head-foot; snout uniquely with a pair of Pomacea canaliculata (adult size up to 75 mm in height) Characteristic pink egg mass, commonly laid on vegetation. distal, long, tentacle-like processes; cephalic tentacles very long. A long 'siphon' is also present. Classification Pomacea canaliculata (Lamarck, 1822) Common name: Golden apple snail Class Gastropoda I nfraclass Caenogastropoda I nformal group Architaenioglossa Order Ampullarida Superfamily Ampullarioidea Family Ampullariidae Genus Pomacea Perry, 1810 Original name: Ampullaria canaliculata Lamarck, 1822. Lamarck, J. B. P. A. de M. de (1822). Histoire naturelle des animaux sans vertèbres Tome sixième.LĘauteur, Paris. 1-232 pp. Type locality: Laguna Guadeloupe ? Santa Fe, Argentina (as ėRivierès de la Guadeloupe) Biology and ecology This species lives on sediment and on aquatic and semi-aquatic vegetation. t lays pink coloured egg masses on plants above the waterline. t has become a major pest of aquatic crops as it eats living plants including rice and taro crops. Distribution ntroduced from South America into the southern United States, East Asia, islands of the ndian Ocean and New Guinea. Notes This pest species has not as yet entered Australia, but ought to be considered a significant risk due to its presence as an invasive in the adjacent ndo-west Pacific region. Two other south Asian ampullariid species have regularly been intercepted by Australian Biosecurity ĕ they are Pila ampullacea (Linnaeus, 1758) and Pila globosa (Swainson, 1822). -

Symbionts and Diseases Associated with Invasive Apple Snails
Symbionts and diseases associated with invasive apple snails Cristina Damborenea, Francisco Brusa and Lisandro Negrete CONICET, División Zoología Invertebrados, Museo de La Plata (FCNyM-UNLP), Paseo del Bosque, 1900 La Plata, Argentina. Email: [email protected], fbrusa@ fcnym.unlp.edu.ar, [email protected] Abstract This contribution summarizes knowledge of organisms associated with apple snails, mainly Pomacea spp., either in a facultative or obligate manner, paying special attention to diseases transmitted via these snails to humans. A wide spectrum of epibionts on the shell and operculum of snails are discussed. Among them algae, ciliates, rotifers, nematodes, flatworms, oligochaetes, dipterans, bryozoans and leeches are facultative, benefitting from the provision of substrate, transport, access to food and protection. Among obligate symbionts, five turbellarian species of the genusTemnocephala are known from the branchial cavity, with T. iheringi the most common and abundant. The leech Helobdella ampullariae also spends its entire life cycle inside the branchial cavity; two copepod species and one mite are found in different sites inside the snails. Details of the nature of the relationships of these specific obligate symbionts are poorly known. Also, extensive studies of an intracellular endosymbiosis are summarized. Apple snails are the first or second hosts of several digenean species, including some bird parasites.A number of human diseases are transmitted by apple snails, angiostrongyliasis being the most important because of the potential seriousness of the disease. Additional keywords: Ampullariidae, Angiostrongylus, commensals, diseases, epibionts, parasites, Pomacea, symbiosis 73 Introduction The term “apple snail” refers to a number of species of freshwater snails belonging to the family Ampullariidae (Caenogastropoda) inhabiting tropical and subtropical regions (Hayes et al., 2015). -

Fecundity of the Chinese Mystery Snail in a Nebraska Reservoir
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Nebraska Cooperative Fish & Wildlife Research Nebraska Cooperative Fish & Wildlife Research Unit -- Staff Publications Unit 2013 Fecundity of the Chinese mystery snail in a Nebraska reservoir Bruce J. Stephen University of Nebraska-Lincoln, [email protected] Craig R. Allen University of Nebraska-Lincoln, [email protected] Noelle M. Chaine University of Nebraska-Lincoln, [email protected] Kent A. Fricke University of Nebraska-Lincoln Danielle M. Haak University of Nebraska-Lincoln, [email protected] See next page for additional authors Follow this and additional works at: https://digitalcommons.unl.edu/ncfwrustaff Part of the Aquaculture and Fisheries Commons, Environmental Indicators and Impact Assessment Commons, Environmental Monitoring Commons, Natural Resource Economics Commons, Natural Resources and Conservation Commons, and the Water Resource Management Commons Stephen, Bruce J.; Allen, Craig R.; Chaine, Noelle M.; Fricke, Kent A.; Haak, Danielle M.; Hellman, Michelle L.; Kill, Robert A.; Nemec, Kristine T.; Pope, Kevin L.; Smeenk, Nicholas A.; Uden, Daniel R.; Unstad, Kody M.; VanderHam, Ashley E.; and Wong, Alec, "Fecundity of the Chinese mystery snail in a Nebraska reservoir" (2013). Nebraska Cooperative Fish & Wildlife Research Unit -- Staff Publications. 121. https://digitalcommons.unl.edu/ncfwrustaff/121 This Article is brought to you for free and open access by the Nebraska Cooperative Fish & Wildlife Research Unit at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Nebraska Cooperative Fish & Wildlife Research Unit -- Staff Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Authors Bruce J. Stephen, Craig R. Allen, Noelle M. Chaine, Kent A. -

Pomacea Canaliculata) Behaviors in Different Water Temperature Gradients
water Article Comparison of Invasive Apple Snail (Pomacea canaliculata) Behaviors in Different Water Temperature Gradients Mi-Jung Bae 1, Eui-Jin Kim 1 and Young-Seuk Park 2,* 1 Nakdonggang National Institute of Biological Resources, Sangju 37242, Korea; [email protected] (M.-J.B.); [email protected] (E.-J.K.) 2 Department of Biology, Kyung Hee University, Dongdaemun, Seoul 02447, Korea * Correspondence: [email protected]; Tel.: +82-2-961-0946 Abstract: Pomacea canaliculata (known as invasive apple snail) is a freshwater snail native to South America that was introduced into many countries (including Asia and North America) as a food source or for organic farming systems. However, it has invaded freshwater ecosystems and become a serious agricultural pest in paddy fields. Water temperature is an important factor determining behavior and successful establishment in new areas. We examined the behavioral responses of P. canaliculata with water temperature changes from 25 ◦C to 30 ◦C, 20 ◦C, and 15 ◦C by quantifying changes in nine behaviors. At the acclimated temperature (25 ◦C), the mobility of P. canaliculata was low during the day, but high at night. Clinging behavior increased as the water temperature decreased from 25 ◦C to 20 ◦C or 15 ◦C. Conversely, ventilation and food consumption increased when the water temperature increased from 25 ◦C to 30 ◦C. A self-organizing map (an unsupervised artificial neural network) was used to classify the behavioral patterns into seven clusters at different water temperatures. These results suggest that the activity levels or certain behaviors of P. canaliculata vary with the water temperature conditions. -

Unsuuseuracsbe
StRd Opelika 85 Junction City HARRIS StRte 96 Geneva StRte 90 96 37 s te e 1 ran TALBOT tR t te S tR e y S V w DISTRICT e 96 Fort Valley 2 Montrose k t 1 S P tR te 96 1 S StR (M TWIGGS e t on Rd iami Valley Rd t R Mac ) R 6 t 2 d Reynolds e 9 S Dublin 9 8 StRt StRte 80 96 StRte 96 Smiths 80 8 PEACH LEE 2 lt Butler 9 S 1 A tR 4 319 7 e t t e StRte 112 2 e MACON t Dudley y DISTRICT 2 R Armour Rd w TAYLOR t R (EmRd 200) SH t StRte 278 Bibb U 4 7 S TAYLOR S 16 0 3 City Upatoi Cr 1 129 11 e t R S t t S 109th Congress of the United StatesR StRte 112 t 32nd (EmRd 200) e MUSCOGEE 3 Phenix G St Reese Rd 6 3 o 2 2 8 Edgewood Rd l 1 e City Forest Rd d 1 Rt e t COLUMBUS 127 e S n t StRte R I t Steam Mill Rd s S Wickham Dr l e Columbus Marshallville 341 s StR te H S w te 2 t R tR Dexter Ladonia Merval Rd 1 te S 1 7 te 127 S y V 185 2 t Rt tRt e 247 ic 2nd Armored Division Rd 7 tR e 127 S t (S o ) S t 0 137 Rte 90) S r Wolf Cr t 57 y 4 d S Perry Rte 2 Upatoi Cr 2 R D tR r e e t t i StRte 41 StRte e 9 StRte n 0 R 23 t n S 126 t S o StRte 6 R StRte 117 R 2 t ( (Airp 1 ) e Rentz o Rd Chester 27 Fort Benning Military Res rt 3 StRte 128 Whitson Rd 4 Cochran 3 22 8 te R TAYLOR Ideal t CHATTAHOOCHEE S MARION StRte 117 StR USHwy 441 Fort Benning te 9 S 0 StRte 26 7 South t Rte 19 129 BLECKLEY 5 Cadwell 13 7 2 7 te 1 RUSSELL StRte 2 StRte 49 HOUSTON tR 1 40 P S e Buena Vista er t StR ry tR te 26 Hwy S S StRt Cusseta tR e 2 te Oglethorpe 6 ( oad 9 26 Montezuma Fire R 00) B u r S n t R t StRte 126 6 B 2 te DISTRICT r S e ) 3 g Hawkinsville t t e R StR 9 r 2 9 -

Occurrence of the Chinese Mystery Snail, Cipangopaludina Chinensis
BioInvasions Records (2016) Volume 5, Issue 3: 149–154 Open Access DOI: http://dx.doi.org/10.3391/bir.2016.5.3.05 © 2016 The Author(s). Journal compilation © 2016 REABIC Rapid Communication Occurrence of the Chinese mystery snail, Cipangopaludina chinensis (Gray, 1834) (Mollusca: Viviparidae) in the Saint John River system, New Brunswick, with review of status in Atlantic Canada Donald F. McAlpine1,*, Dwayne A. W. Lepitzki2, Frederick W. Schueler3, Fenning J.T. McAlpine1, Andrew Hebda4, Robert G. Forsyth1, Annegret Nicolai5, John E. Maunder6 and Ron G. Noseworthy7 1New Brunswick Museum, 277 Douglas Avenue, Saint John, New Brunswick, E2K 1E5 Canada 2Wildlife Systems Research, P.O. Box 1311, Banff, Alberta, T1L 1B3 Canada 3RR # 2, Bishops Mills, Ontario, K0G 1T0 Canada 4Nova Scotia Museum of Natural History, 1747 Summer Street, Halifax, Nova Scotia, B3H 3A6 Canada 5UMR-CNRS 6553 EcoBio, Campus Beaulieu, Université Rennes 1, 35042 Rennes cedex, France 6P.O. Box 250, Pouch Cove, Newfoundland and Labrador, A0A 3L0 Canada 7School of Marine Biomedical Science, Jeju National University, Jeju 690-756, Republic of Korea *Corresponding author E-mail: [email protected] Received: 27 February 2016 / Accepted: 1 July 2016 / Published online: 20 July 2016 Handling editor: Carles Alcaraz Abstract The Chinese mystery snail, Cipangopaludina [=Bellamya] chinensis, is documented for the first time in the Saint John River, New Brunswick, a watercourse which drains the largest watershed in Atlantic Canada. This is the first non-native mollusc known to be established in the Saint John River system. Although significant ecosystem effects of the species seem unlikely, possible introduction of C.