PDF Download
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WHO Drug Information Vol. 12, No. 3, 1998
WHO DRUG INFORMATION VOLUME 12 NUMBER 3 • 1998 RECOMMENDED INN LIST 40 INTERNATIONAL NONPROPRIETARY NAMES FOR PHARMACEUTICAL SUBSTANCES WORLD HEALTH ORGANIZATION • GENEVA Volume 12, Number 3, 1998 World Health Organization, Geneva WHO Drug Information Contents Seratrodast and hepatic dysfunction 146 Meloxicam safety similar to other NSAIDs 147 Proxibarbal withdrawn from the market 147 General Policy Issues Cholestin an unapproved drug 147 Vigabatrin and visual defects 147 Starting materials for pharmaceutical products: safety concerns 129 Glycerol contaminated with diethylene glycol 129 ATC/DDD Classification (final) 148 Pharmaceutical excipients: certificates of analysis and vendor qualification 130 ATC/DDD Classification Quality assurance and supply of starting (temporary) 150 materials 132 Implementation of vendor certification 134 Control and safe trade in starting materials Essential Drugs for pharmaceuticals: recommendations 134 WHO Model Formulary: Immunosuppressives, antineoplastics and drugs used in palliative care Reports on Individual Drugs Immunosuppresive drugs 153 Tamoxifen in the prevention and treatment Azathioprine 153 of breast cancer 136 Ciclosporin 154 Selective serotonin re-uptake inhibitors and Cytotoxic drugs 154 withdrawal reactions 136 Asparaginase 157 Triclabendazole and fascioliasis 138 Bleomycin 157 Calcium folinate 157 Chlormethine 158 Current Topics Cisplatin 158 Reverse transcriptase activity in vaccines 140 Cyclophosphamide 158 Consumer protection and herbal remedies 141 Cytarabine 159 Indiscriminate antibiotic -

Bitamoazedthesis.Pdf (2.274Mb)
GASTROINTESTINAL ABSORPTION OF HEPARINS A Dissertation Submitted to the College of Graduate Studies and Research in Partial Fulfillments of the Requirements for the Degree of Doctor of Philosophy in the Graduate Program of Veterinary Biomedical Sciences University of Saskatchewan Saskatoon Bita Moazed Copyright Bita Moazed, November 2009. All Rights Reserved PERMISSION TO USE In presenting this dissertation in partial fulfillment of the requirements for a Doctor of Philosophy degree from the University of Saskatchewan, I agree that the Libraries of this University may make it freely available for inspection. I further agree that permission for copying of this thesis/dissertation in any manner, in whole or in part, for scholarly purposes may be granted by Dr. Linda M. Hiebert who supervised my dissertation work or, in her absence, by the chair of the Graduate Program of Veterinary Biomedical Sciences. It is understood that any copying or publication or use of this dissertation or parts thereof for financial gain shall not be allowed without my written permission. It is also understood that due recognition shall be given to me and the University of Saskatchewan in any scholarly use which may be made of any material in this dissertation. Requests for permission to copy or to make other use of other material in this dissertation in whole or part should be addressed to: Chair of the Graduate Program of Veterinary Biomedical Sciences Western College of Veterinary Medicine University of Saskatchewan 52 Campus Drive Saskatoon, Saskatchewan S7N 5B4 Canada i PREFACE This dissertation has been organized as a series of manuscripts that was or will be submitted for publication in scientific journals. -

Package Leaflet: Information for the User Innohep® 10,000 IU/Ml Solution for Injection Tinzaparin Sodium
Package leaflet: Information for the user innohep® 10,000 IU/ml solution for injection tinzaparin sodium Read all of this leaflet carefully before you start using this medicine because it contains important information for you. • Keep this leaflet. You may need to read it again. • If you have any further questions, ask your doctor, pharmacist or nurse. • This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours. • If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4. • In this leaflet innohep 10,000 IU/ml will be called innohep. What is in this leaflet 1. What innohep® is and what it is used for 2. What you need to know before you use innohep® 3. How to use innohep® 4. Possible side effects 5. How to store innohep® 6. Contents of the pack and other information 1. What innohep® is and what it is used for innohep is a type of heparin – a low molecular weight heparin – and belongs to a group of medicines called anticoagulants; these medicines affect how your blood clots. innohep prevents clotting, allowing normal blood flow through the arteries and veins. innohep is used to: Prevent blood clots in adults before and after an operation. Prevent blood clots in adults who have an increased risk of blood clots e.g. due to an acute illness with limited mobility. Prevent blood clots being formed in haemodialysis equipment in patients undergoing haemodialysis or haemofiltration. -

Review of Anticoagulant Drugs in Paediatric Thromboembolic Disease
18th Expert Committee on the Selection and Use of Essential Medicines (21 to 25 March 2011) Section 12: Cardiovascular medicines 12.5 Antithrombotic medicines Review of Anticoagulant Drugs in Paediatric Thromboembolic Disease September 2010 Prepared by: Fiona Newall Anticoagulation Nurse Manager/ Senior Research Fellow Royal Children’s Hospital/ The University of Melbourne Melbourne, Australia 1 Contents Intent of Review Review of indications for antithrombotic therapy in children Venous thromboembolism Arterial thrombeombolism Anticoagulant Drugs Unfractionated heparin Mechanism of action and pharmacology Dosing and administration Monitoring Adverse events Formulary Summary recommendations Vitamin K antagonists Mechanism of action and pharmacology Dosing and administration Monitoring Adverse events Formulary Summary recommendations Low Molecular Weight Heparins Mechanism of action and pharmacology Dosing and administration Monitoring Adverse events Formulary Summary recommendations ??Novel anticoagulants Summary 2 1. Intent of Review To review the indications for anticoagulant therapies in children. To review the epidemiology of thromboembolic events in children. To review the literature and collate the evidence regarding dosing, administration and monitoring of anticoagulant therapies in childhood. To review the safety of anticoagulant therapies and supervision required To give recommendations for the inclusion of anticoagulants on the WHO Essential Medicines List 2. Review of indications for antithrombotic therapy in children Anticoagulant therapies are given for the prevention or treatment of venous and/or arterial thrombosis. The process of ‘development haemostasis’, whereby the proteins involved in coagulation change quantitatively and qualitatively with age across childhood, essentially protects children against thrombosis(1-5). For this reason, insults such as immobilization are unlikely to trigger the development of thrombotic diseases in children. -

SPECIALTY MEDICATIONS Available Through Accredo Health Group, Inc., Medco’S Specialty Pharmacy Call Toll-Free (800) 803-2523, 8:00 A.M
SPECIALTY MEDICATIONS available through Accredo Health Group, Inc., Medco’s specialty pharmacy Call toll-free (800) 803-2523, 8:00 a.m. to 8:00 p.m., eastern time, Monday through Friday, to confirm that your medication is covered. Effective as of July 1, 2011 Abraxane® (paclitaxel protein-bound particles) Berinert® (C 1 esterase inhibitor [human])* (PA) (QD) Actemra ™ (tocilizumab) (PA) Betaseron® (interferon beta-1b) (PA) Actimmune® (interferon gamma-1b) (PA) Botox® (botulinum toxin type A) (PA) Adagen® (pegademase bovine) Carbaglu ™ (carglumic acid) Adcirca® (tadalafil) (ST) (QD) Carimune® NF (immune globulin intravenous [human]) (PA) Advate® (antihemophilic factor [recombinant]) (CPA) Cerezyme® (imiglucerase) (CPA) (ST) Afinitor® (everolimus) (PA) (QD) Cimzia® (certolizumab pegol) (ST) Aldurazyme® (laronidase) (CPA) Copaxone® (glatiramer acetate) (PA) Alphanate® (antihemophilic factor [human]) (CPA) Copegus® (ribavirin) (ST) AlphaNine® SD (coagulation factor IX [human]) (CPA) Corifact® (factor XIII [human]) (CPA) Amevive® (alefacept) (PA) Cystadane® (betaine) Ampyra ™ (dalfampridine) (PA) CytoGam® (cytomegalovirus immune globulin Apokyn® (apomorphine hydrochloride) (PA) (QD) intravenous [human])* (CPA) Aralast® (alpha[1]-proteinase inhibitor [human]) Cytovene® IV (ganciclovir sodium)* Aranesp® (darbepoetin alfa) (PA) Dacogen® (decitabine) Arcalyst® (rilonacept) (PA) (QD) Dysport® (abobotulinumtoxinA) (PA) Arixtra® (fondaparinux sodium)* Egrifta ™ (tesamorelin) (PA) Arranon® (nelarabine) Elaprase® (idursulfase) (CPA) Arzerra® (ofatumumab) -

Hemosil ® Liquid Anti-Xa
H E M O S I L® LIQUID ANTI-XA Measuring heparin and apixaban: Simple, fast, 24/7 • Liquid formulation, ready-to-use • One-stage, chromogenic anti-Xa assay • Universal calibration for unfractionated heparin (UFH) and low molecular weight heparin (LMWH) • Drug specific calibrators and controls for measurement of apixaban Measuring heparin and apixaban Unfractionated and low molecular weight heparin Heparin is a highly sulfated polysaccharide Laboratory monitoring is extremely important to characterized by a wide molecular weight range and assess the appropriate level of anticoagulation in potent anticoagulant activity. It exists either as UFH patients receiving UFH. Anti-Xa is recommended for or as depolymerized LMWH. UFH and LMWH have measuring both UFH and LMWH. a rapid anticoagulant effect and are used in the prevention and treatment of venous thrombosis and Anti-Xa testing for measuring UFH helps improve acute coronary syndrome. quality of care and patient experience while reducing costs, when compared with APTT testing.1 UFH and LMWH anticoagulant activity occurs when The advantages include: a complex with antithrombin (AT) is formed, • Higher precision potentiating its anticoagulant activity up to • Lower levels of discordant results1,2,4 1,000-fold, which inactivates both thrombin (FIIa) • Faster time to achieve therapeutic levels1,3,4 and Factor Xa (FXa). UFH acts through both FIIa 1,3,4,5 and FXa inhibition, while LMWH is a more efficient • Fewer tests and dosage changes catalyst for FXa inhibition. Direct Xa inhibitors Anticoagulation for patients with venous DOACs do not require routine monitoring. However, thromboembolism (VTE) previously included there are specific instances when an understanding heparin, heparin derivatives and/or oral vitamin K of the DOAC concentration in a patient sample may antagonists. -

Pharmacokinetics of Anticoagulant Rodenticides in Target and Non-Target Organisms Katherine Horak U.S
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln USDA National Wildlife Research Center - Staff U.S. Department of Agriculture: Animal and Plant Publications Health Inspection Service 2018 Pharmacokinetics of Anticoagulant Rodenticides in Target and Non-target Organisms Katherine Horak U.S. Department of Agriculture, [email protected] Penny M. Fisher Landcare Research Brian M. Hopkins Landcare Research Follow this and additional works at: https://digitalcommons.unl.edu/icwdm_usdanwrc Part of the Life Sciences Commons Horak, Katherine; Fisher, Penny M.; and Hopkins, Brian M., "Pharmacokinetics of Anticoagulant Rodenticides in Target and Non- target Organisms" (2018). USDA National Wildlife Research Center - Staff Publications. 2091. https://digitalcommons.unl.edu/icwdm_usdanwrc/2091 This Article is brought to you for free and open access by the U.S. Department of Agriculture: Animal and Plant Health Inspection Service at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in USDA National Wildlife Research Center - Staff ubP lications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Chapter 4 Pharmacokinetics of Anticoagulant Rodenticides in Target and Non-target Organisms Katherine E. Horak, Penny M. Fisher, and Brian Hopkins 1 Introduction The concentration of a compound at the site of action is a determinant of its toxicity. This principle is affected by a variety of factors including the chemical properties of the compound (pKa, lipophilicity, molecular size), receptor binding affinity, route of exposure, and physiological properties of the organism. Many compounds have to undergo chemical changes, biotransformation, into more toxic or less toxic forms. Because of all of these variables, predicting toxic effects and performing risk assess- ments of compounds based solely on dose are less accurate than those that include data on absorption, distribution, metabolism (biotransformation), and excretion of the compound. -

Doacs) and the Antidote Idarucizumab
Update overview 2019 of reports on direct oral anticoagulants (DOACs) and the antidote idarucizumab Introduction Lareb previously published yearly overviews of reports (most recently in 2018) in consultation with the Medicines Evaluation Board CBG-MEB, concerning the direct oral anticoagulants (DOACs) dabigatran Pradaxa®, registered in the Netherlands in 2008 [1], rivaroxaban Xarelto®, registered in 2008 [2], apixaban Eliquis®, registered in 2011 [3] and edoxaban (Lixiana®), registered in 2015 [4-9]. The current overview provides a new yearly update of the reports received by Lareb for these DOACs. Furthermore, reports received by Lareb for the antidote idarucizumab are described in this overview. Idarucizumab is a specific antidote for dabigatran and was registered in the Netherlands in 2015 [10]. For this overview, data from the national ADR database were used. These data include reports with serious outcome from the Lareb Intensive Monitoring System (LIM). The DOACs have been monitored with the LIM methodology since September 2012. Prescription data The number of patients using DOACs in the Netherlands according to the GIP database is shown in table 1 [11]. These data are based on extramurally provided medication included in the Dutch health insurance package. Because the antidote idarucizumab is administered in the hospital and not reimbursed directly via the healthcare insurance, these data are not available for this drug. The number of reports received by the Netherlands Pharmacovigilance Centre Lareb per year since 2013 for each DOAC, is also shown in table 1. Furthermore, table 1 shows the calculated number of these reports per 1.000 users according to the GIP database. As noted before, the data from the GIP database represent reimbursed medicines and these may differ from actually prescribed medicines. -

Dabigatran, Rivaroxaban, and Warfarin in the Oldest Adults With
CLINICAL INVESTIGATION Dabigatran, Rivaroxaban, and Warfarin in the Oldest Adults with Atrial Fibrillation in Taiwan Chao-Lun Lai, MD, PhD,*†‡§ Ho-Min Chen, MS,† Min-Tsun Liao, MD,* and Ting-Tse Lin, MD* mortality and cardiovascular mortality than those who OBJECTIVES: To compare the effectiveness and safety of used warfarin. Reduced-dose dabigatran was also associ- reduced-dose dabigatran, reduced-dose rivaroxaban, and warfa- ated with lower risk of intracranial hemorrhage than war- rin in individuals aged 85 and older with atrial fibrillation (AF). farin. J Am AmGeriatr GeriatrSoc Soc66:1567–1574, 2018. 2018. DESIGN: Retrospective cohort study. SETTING: Taiwan National Health Insurance claims database, 20112015. Key words: dabigatran; rivaroxaban; warfarin; effec- PARTICIPANTS: Individuals with AF aged 85 and older tiveness; safety; octogenarian (mean 88.6) with incident use of oral anticoagulants between June 1, 2012 and May 31, 2015 (N54,722; dabi- gatran 110 mg, n51,489; rivaroxaban 15 mg/10 mg, n51,736; warfarin, n51,497). MEASUREMENTS: Clinical outcomes included all-cause death, cardiovascular death, ischemic stroke, acute myocardial he risk of ischemic stroke is 5 times as high in individ- infarction, arterial embolism or thrombosis, intracranial hem- T uals with atrial fibrillation (AF) than in those with- orrhage, and gastrointestinal hemorrhage needing transfusion. out.1 Warfarin, the classic vitamin K antagonist, can reduce 2 Propensity score–matched analysis was performed, and the the risk of ischemic stroke by approximately 60%, but the marginal proportional hazards model was used to estimate the narrow therapeutic window and risk of bleeding complica- relative risk of various clinical outcomes in a matched tions associated with warfarin therapy have led to its being 1 dabigatran-warfarin cohort (n51,180 in each group) and a underused. -

Low Molecular Weight Heparins and Heparinoids
NEW DRUGS, OLD DRUGS NEW DRUGS, OLD DRUGS Low molecular weight heparins and heparinoids John W Eikelboom and Graeme J Hankey UNFRACTIONATED HEPARIN has been used in clinical ABSTRACT practice for more than 50 years and is established as an effective parenteral anticoagulant for the prevention and ■ Several low molecular weight (LMW) heparin treatment of various thrombotic disorders. However, low preparations, including dalteparin, enoxaparin and molecularThe Medical weight Journal (LMW) of heparinsAustralia haveISSN: recently 0025-729X emerged 7 October as nadroparin, as well as the heparinoid danaparoid sodium, more2002 convenient, 177 6 379-383 safe and effective alternatives to unfrac- are approved for use in Australia. 1 tionated©The heparin Medical (BoxJournal 1). of AustraliaIn Australia, 2002 wwwLMW.mja.com.au heparins are ■ LMW heparins are replacing unfractionated heparin for replacingNew Drugs,unfractionated Old Drugs heparin for preventing and treating the prevention and treatment of venous thromboembolism venous thromboembolism and for the initial treatment of and the treatment of non-ST-segment-elevation acute unstable acute coronary syndromes. The LMW heparinoid coronary syndromes. danaparoid sodium is widely used to treat immune heparin- ■ induced thrombocytopenia. The advantages of LMW heparins over unfractionated heparin include a longer half-life (allowing once-daily or twice-daily subcutaneous dosing), high bioavailability and Limitations of unfractionated heparin predictable anticoagulant response (avoiding the need -
For National Autllority Use Only
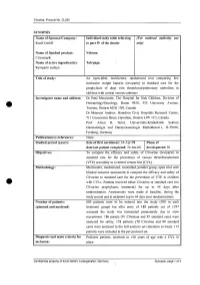
Clivarine. Protocol No. CL055 SYNOPSIS Name of Sponsor/Company: Individual study table referring (For national autllOrity use Knoll GmbH to part IV of the dossier only) Name of finished product: Volume: Clivarine® Name of active ingredient(s): Tab/page: Reviparin sodium Title of study: An open-label, multicentre, randomized trial comparing low molecular weight heparin (reviparin) to standard care for the prophylaxis of deep vein thrombosis/pulmonary embolism in children with central venous catheters Investigator name and address: Dr Patti Massicotte, The Hospital for Sick Children, Division of Hematology/Oncology, Room 9420, 555 University Avenue, Toronto, Ontario M5G JX8, Canada Dr Maureen Andrew, Hamilton Civic Hospitals Research Center, 711 Concession Street, Hamilton, Ontario L8V JC3, Canada Prof. Anton H. Sutor, Universitats-Kinderklink Sektion Haematologie und Haemostaseologie Mathildenstr.l, D-79106, Freiburg, Germany Publication(s) (reference): None. Studied period (years): date of first enrolment: 24-Apr-98 Phase of date' last patient completed: 24-Jan-00 development: 111 Objectives: To compare the efficacy and safety of Clivarine (reviparin) to standard care for the prevention of venous thromboembolism (VTE) secondary to a central venous line (CVL). Methodology: Multicentre, randomized, controlled, parallel group, open trial with blinded outcome assessment to compare the efficacy and safety of Clivarine to standard care for the prevention of VTE in children e· with CVLs. Patients received either Clivarine or standard care (no -

Edoxaban Switch Programme - Frequently Asked Questions
Edoxaban Switch Programme - Frequently Asked Questions What should I tell patients? NHS Tayside is reviewing all patients currently receiving a Direct Oral Anticoagulant (DOAC) for stroke prevention in NV-AF(non-valvular atrial fibrillation) Edoxaban has been identified as the first choice DOAC. It is similarly effective to the other DOAC options but costs considerably less Clinical experts in Tayside are supporting the use of edoxaban All newly diagnosed NV-AF patients will be started on edoxaban as 1st choice for those unsuitable for warfarin Existing patients already on a DOAC for NV-AF are to be reviewed and considered for switch to edoxaban This will help to ensure that the money available to spend on medicines is being used appropriately Is edoxaban as good as the other DOACs? Yes, the evidence is that it is as effective as warfarin and the other DOACs. It is licensed for this indication and has been recommended by the Scottish Medicines Consortium Other Scottish Health Boards are also considering the use of edoxaban A recent Health Improvement Scotland (HIS) summary (http://www.healthcareimprovementscotland.org/our_work/cardiovascular_dis ease/programme_resources/doac_review_report.aspx) is consistent with this Tayside switch recommendation Lead clinicians from cardiology, stroke, vascular medicine, relevant MCN’s and haematology are all supportive of this Tayside guidance on the basis of current evidence Will we need to do a further switch if the price of other DOACs falls? The rebate on edoxaban is in place for five