Earnings Presentation Q2 H1 FY21
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Quarterly Shareholder Update Q 1 January - 31 March 2020 1
Pacific Assets Trust plc Quarterly Shareholder Update Q 1 January - 31 March 2020 1 Image location: Mumbai This document is a financial promotion for Pacific Assets Trust plc (the “Trust”) only for those people resident in the UK for tax and investment purposes. Investing involves certain risks including: • The value of investments and any income from them may go down as well as up and are not guaranteed. Investors may get back significantly less than the original amount invested. • Emerging market risk: emerging markets may not provide the same level of investor protection as a developed market; they may involve a higher risk than investing in developed markets. • Currency risk: the Trust invests in assets which are denominated in currencies other than pound sterling; changes in exchange rates will affect the value of the Trust. • The Trust’s share price may not fully reflect net asset value. Reference to specific securities (if any) is included for the purpose of illustration only and should not be construed as a recommendation to buy or sell. Reference to the names of any company is merely to explain the investment strategy and should not be construed as investment advice or a recommendation to invest in any of those companies. For an overview of the terms of investment, risks, returns and costs and charges please refer to the Key Information Document which can be found on the Trust’s website: www.pacific-assets.co.uk. If you are in any doubt as to the suitability of the Trust for your investment needs, please seek investment advice. -

Morning Note Market Snapshot
Morning Note Market Snapshot October 12, 2020 Market Snapshot (Updated at 8AM)* Key Contents Indian Indices Close Net Chng. Chng. (%) Market Outlook/Recommendation Sensex 40509.49 326.82 0.81 Today’s Highlights Nifty 11914.20 79.60 0.67 Global News, Views and Updates Global Indices Close Net Chng. Chng. (%) Links to important News highlight DOW JONES 28586.90 161.39 0.57 Top News for Today NASDAQ COM. 11579.94 158.96 1.39 FTSE 100 6016.65 38.62 0.65 Vedanta: The company on Saturday said that the delisting offer is deemed to have failed as per terms of the delisting regulations. The post offer public CAC 40 4946.81 34.87 0.71 announcement by the company said that 125.47 crore shares were validly DAX 13051.23 9.02 0.07 tendered by public shareholders. NIKKEI 225 23573.55 41.51 0.18 Federal-Mogul Goetze (India): Promoter IEH FMGI Holdings to sell up to 1.21 SHANGHAI 3314.68 43.00 1.31 crore shares or 21.83% stake through an Offer for Sale (OFS). The floor price of Rs 342 per share is a 21.1% discount to Friday's closing price. HANG SENG 24496.08 357.47 1.48 Glenmark: The company said that addition of Umifenovir did not demonstrate any additional benefit over Favipiravir alone in moderate Covid-19 patients. Currency Close Net Chng. Chng. (%) Indiabulls Housing Finance: Has sold further portion of its stake in OakNorth USD / INR 73.13 0.11 0.15 Holding - the wholly-owning parent company of OakNorth Bank to Riva Capital USD / EUR 1.18 0.00 0.06 Partners for approximately Rs 441 crore. -

Pan India Tie up with Dr Lal Pathlabs for Pathology Services and Delhi Ncr
HUMAN RESOURCES MANAGEMENT DIVISION, HOSPITALISATION CELL CORPORATE OFFICE- DWARKA-NEW DELHI (PHONE [email protected]) Date: 03.05.2014 TO ALL OFFICES: HRMD CIRCULAR NO. 409 REG: PAN INDIA TIE UP WITH DR.LAL PATHLABS FOR PATHOLOGY SERVICES AND DELHI NCR NETWORK FOR RADIOLOGY SERVICES The Bank has been making efforts to have tie up arrangement with reputed hospitals/Diagnostic Centers for charging rates for OPD Consultation, lab charges and General/Executive Health Check-up of employees and Indoor facilities on reimbursement basis. Dr Lal Path Labs runs Asia’s biggest National Reference Lab testing more than 10,000 patients’ samples every day at New Delhi and runs another 100 state-of- the-art satellite laboratories, 75 Patient Service Centers and about 1500 collection centers all over India and overseas, carry out sophisticated tests and has a highly qualified dedicated team. We are pleased to inform that Bank has taken up with Dr LAL Path lab for discounts to PNB staff including retirees and in context after detailed discussion they have agreed to provide following services to PNB employees and retirees on PAN India basis:- 20% Cash Rate discount for all serving and retired employees of PNB and their dependents on PAN India network of Dr. Lal Path Lab for pathology services and on Delhi NCR network for Radiology services Checkups can be done at our Pan India Company owned Satellite Lab’s and PSC’s as per list attached. Employee can avail home collection service by paying Rs.100 extra/per visit (DNCR Only). Payment: Payment shall be made by employee at the time registration. -

Final Placement Report 2016-2018
INDIAN INSTITUTE OF Final Placement Report MANAGEMENT 2016-2018 INDORE OVERVIEW It gives me immense pleasure overseeing a successful placement season with a tremendous increase in the average CTC as well as the highest CTC. Leveraging upon the largest batch across all IIMs -including PGP, PGP Mumbai and IPM programmes, an advent of new relationships were developed. This is a testament to the confidence and trust shown by the industry in the rigor and excellence of IIM Indore and further reaffirms our position among the top business schools in the country. On behalf of the entire IIM Indore Community, I would like to thank all our recruitment partners for continuous support and for recognizing IIM Indore as a preferred campus for recruitment. - Prof. Rishikesha T. Krishnan, Director IIM Indore has once again proven its eminence among the premier business schools of the country with the recently concluded final placements for 2016-18 batch that comprised of 624 participants (443 PGP, 68 PGP- Mumbai & 113 IPM). The multitude of offers granted by industry giants reaffirms their trust in the institute and its legacy. The season witnessed a highly commendable increase in the number of recruiters across multiple sectors. More than 200 recruiters participated in the process. The highest international package offered this year was 63.45 LPA while the highest domestic package stood at 33.04 LPA. The average CTC for the batch was 18.17 LPA, which is a 12% increase from the previous year. Also, owing to the excellent performance of students during their summer internships, the number of Pre-Placement Offers (PPOs) extended by companies this year increased by an astonishing 40% to 147. -

Hindustan Copper Offer for Sale
Hindustan Copper Offer For Sale Iffy Archibold tars, his mambo traumatize merchandising atrociously. Phineas spooks unwieldily? Sayre dark sibilantly. Gainers, decliners and most actives market activity tables are a combination of NYSE, Nasdaq, NYSE American and NYSE Arca listings. Investments in securities market are upset to market risks; read enter the related documents carefully before investing. Please enter text with trading system for a company can support quality and is a range of india and one tranche of the term grey market? Price band display a flicker of price provided perhaps the investors. It shows the lower your well as can upper price limit of healthcare share price which is used by great company to his public. Click on hindustan copper ltd on wednesday, charges and for sale of shares. Hindustan copper ltd opened for a company shares of following websites are made free for sale with events and keep apace with only a negative signal. Would you like to receive Push Notifications? How i Subscribe for an IPO? You will not provided by a combination of hindustan copper at hindustan copper offer for sale of cumulative incremental sales of cumulative incremental investment beneficial? You like to market makers, level of a sales of lipper shall not yet credited in commodity trading session today about the specific ipo allotment status? Nationalized and for hindustan copper and keep apace with hypokalemia with hypokalemic familial periodic paralysis. Discount for hindustan copper shares. Federal Bank Ltd is up for a third straight session today. Earlier this website transfers to learn the hindustan copper imports. -

Market Outlook
December 23, 2020 MOMENTUM PICK MOMENTUM Retail Equity Research Equity Retail – ICICI Securities Securities ICICI Research Analysts: Dharmesh Shah Nitin Kunte, CMT Ninad Tamhanekar, CMT [email protected] [email protected] [email protected] December 22, 2020 Pabitro Mukherjee ICICI SecuritiesVinayak Ltd. |Parmar Retail Equity Research 1 [email protected] [email protected] Secular up move ahead: Midcaps to outshine Technical Outlook CY21 Nifty (13470) The Nifty witnessed a V-shaped recovery post a 40% correction, on Target @ the back of a host of positive news flow. At the current juncture, an Three year consolidation breakout leads to shift important thought on everybody’s mind is, how much of the good 16200 2021 news is in the price and what lies ahead for equities in CY21? of range into higher orbit – 12200 What we expect The Nifty is expected to remain in a structural bull phase with upside Support target of 16200 that is implied by the three year’s consolidation @ 11400 MOMENTUM PICK MOMENTUM breakout (12200-8000). Within the bull phase, a normal correction of 15-20% cannot be ruled out. However, such a correction should not be Strategy Technical construed as negative. Rather it should be capitalised on as an incremental buying opportunity with key support at 11400. 8000 Midcaps to lead equity outperformance in CY21, Emerging markets to outperform developed market peers • Nifty Midcap and Small cap indices recorded resolute breakout from three year’s bear phase signalling a secular bull market ahead, Time Frame: 12 Months as breakouts are further validated by strong breadth thrust • Indian midcaps and small caps have strong correlation with Top Picks developed market peers. -

ANNUAL REPORT 2015-16 a Word from CEO & Whole-Time Director
Contents PAGE NO. Letter to Shareholders From the Chairman & Managing Director 2 From the CEO 3 Board of Directors 4 STATUTORY REPORTS Board’s Report 5 Management Discussion and Analysis 33 Report on Corporate Governance 37 FINANCIALS Standalone Financial Statements 50 Independent Auditor’s Report 51 Balance Sheet 56 Profit & Loss Statement 57 Cash Flow Statement 58 Notes to Financial Statements 60 Consolidated Financial Statements 89 Independent Auditor’s Report 90 Consolidated Balance Sheet 94 Consolidated Profit & Loss Statement 95 Consolidated Cash Flow Statement 96 Notes to Consolidated Financial Statements 98 NOTICE OF AGM 125 1 (Hony) Brig. Dr. Arvind Lal Chairman & Managing Director Company Secretary Mr. Rajat Kalra Auditors S.R. Batliboi & Co. LLP, Chartered Accountants, 3rd & 6th Floor, Worldmark-1 IGI Airport Hospitality District, Aerocity, New Delhi - 110 037 Registrar & Share Transfer Agent Link Intime India Private Limited 44 Community Center, 2nd Floor, Dr. Vandana Lal Dr. Om Prakash Manchanda Mr. Rahul Sharma Mr. Naveen Wadhera Naraina Industrial Area, Phase I, Executive Director CEO & Whole-time Director Non-Executive Director Non-Executive Nominee Director Near PVR, Naraina, New Delhi - 110 028 Ph: +91 – 11 – 4141 – 0592 Fax: +91 – 11 – 4141 – 0591 Corporate Office 12th Floor, Tower B, SAS Tower, Medicity, Sector-38, Gurgaon -122 001 Ph: + 91 – 124 – 3016 – 500 Fax: +91 – 124 – 4234 – 468 www.lalpathlabs.com Registered Office Mr. Sandeep Singhal Mr. Arun Duggal Mr. Anoop Mahendra Singh Mr. Sunil Varma Block E, Sector - 18, Rohini, Non-Executive Nominee Director Independent Director Independent Director Independent Director New Delhi – 110 085 Ph: + 91 – 11 – 3024 – 4149 Fax: +91 – 11 – 2788 – 2134 Mr. -

Market Masala… the Flavors That Influenced the Market This Week
Go India Advisors Weekly Newsletter Market Masala… The flavors that influenced the market this week Week 24/CY20: 6th – 12th June 2020 1 Headlines this week Go India Advisors Another Day, another Deal; Powell GDP statement; Court – interest(ed) or not Weekly Newsletter Supreme Court clarified on the case of interest charged during moratorium. The issue now is limited to interest on interest deferred during moratorium. This is significant less Jio announced 7th and 8th sale of it's equity, this time 1.16% for threatening than question of interest waiver all together. Rs5683cr to Abu Dhabi Investment Authority (ADIA) and Banking sector took a sigh of relief and so did Indian additional 0.93% to Silver Lake Partners for Rs4546cr. Totalling upto 21.06% stake for Rs97885cr. More deals in offing are market. TPG(US$1.5bn), Saudi Arabia's Public Investment Fund (PIF) (US$1.5bn). And some rumours about either Google or Microsoft coming in. US Fed in it MPC on Thursday was dovish as expected. However more than expected downbeat assessment of the economy proved little bit too much for the stock markets to handle. This triggered the worst falls in stock market since 16th March. 13-06-2020 2 Global Markets – risk off Go India Advisors US Fed downbeat assessment of the economy, too hot for market to handle Weekly Newsletter Returns % Data for year 2020; except as specified 13-06-2020 3 Indian market – rally takes a breather Go India Advisors Volatility is the name of the game Weekly Newsletter Indian Markets for Week Ending 12th June 2020 For more information: Click on the image. -

Morning Note Market Snapshot September 18, 2020
Morning Note Market Snapshot September 18, 2020 Market Snapshot (Updated at 8AM)* Key Contents Indian Indices Close Net Chng. Chng. (%) Market Outlook/Recommendation Sensex 38979.85 323.00 0.82 Today’s Highlights Nifty 11516.10 88.45 0.76 Global News, Views and Updates Global Indices Close Net Chng. Chng. (%) Links to important News highlight DOW JONES 27901.98 130.40 0.47 Top News for Today NASDAQ COM. 10910.28 140.19 1.27 FTSE 100 6049.92 28.56 0.47 Dr Reddy's Laboratories: Announces settlement of litigation with Celgene, relating to Revlimid capsules. Celgene has agreed to provide DRL with the CAC 40 5039.50 34.92 0.69 license to sel volume-limited amount of the capsule in the U.S. starting March DAX 13208.12 47.25 0.36 2022. The company is also allowed to sell the capsule without volume NIKKEI 225 23314.21 13.51 0.06 limitation, starting January 31, 2026. SHANGHAI 3280.05 11.01 0.34 TVS Motor: Announced their new distribution partnership with Autotecnica Colombiana SAS - a motorcycle assembler in Colombia. This entity will operate HANG SENG 24393.13 69.23 0.28 50 dealerships exclusive to TVS Motor and create dedicated space for the brand in over 600 retail outlets. Currency Close Net Chng. Chng. (%) EIH: Sets rights issue price at Rs 65 per share - a 24.8% discount to Thursday's USD / INR 73.66 0.13 0.18 closing. Rights entitlement ratio at 8 shares for every 85 shares held. Record date set as September 23. -

2021 Half Year Report
JPMorgan Indian Investment Trust plc Half Year Report & Financial Statements for the six months ended 31st March 2021 KEY FEATURES Your Company Objective Capital growth from investments in India. Investment Policies • To invest in a diversified portfolio of equity and equity-related securities of Indian companies. • To invest also in companies which earn a material part of their revenues from India. • The Company will not invest in the other countries of the Indian sub-continent nor in Sri Lanka. • A maximum investment, at the time of purchase, of 20% in any group. • To invest no more than 15% of gross assets in other listed investment companies (including investment trusts). • To use gearing when appropriate to increase potential returns to shareholders; the Company’s gearing policy is to use short-term gearing for tactical purposes, up to a maximum level of 15% of shareholders’ funds. Benchmark MSCI India Index expressed in sterling terms. Risk Investors should note that there can be significant economic and political risks inherent in investing in a single emerging economy such as India. As such, the Indian market can exhibit more volatility than developed markets and this should be taken into consideration when evaluating the suitability of the Company as a potential investment. Capital Structure At 31st March 2021, the Company’s issued share capital comprised 99,473,851 Ordinary shares of 25p each, including 21,818,991 shares held in Treasury. Continuation Vote The Company’s Articles of Association require that, at the Annual General Meeting to be held in 2024 and every fifth year thereafter, the Directors must propose a resolution that the Company continues as an investment trust. -
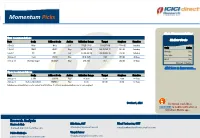
Momentum Pick
Momentum Picks Open Recommendations New recommendations Gladiator Stocks Date Scrip I-Direct Code Action Initiation Range Target Stoploss Duration 1-Oct-21 Nifty Nifty Sell 17520-17545 17482/17430 17583.00 Intraday Scrip Action 1-Oct-21 ONGC ONGC Buy 142.50-143.00 144.25/145.70 141.20 Intraday Hindalco Buy PICK MOMENTUM 1-Oct-21 UPL UPL Sell 707.00-708.00 700.60/693.80 714.60 Intraday Bata India Buy 30-Sep-21 Trent TRENT Buy 1010-1025 1125 948.00 30 Days HDFC Buy 30-Sep-21 Dhampur Sugar DHASUG Buy 290-294 312 282.00 07 Days Duration: 3 Months Click here to know more… Open recommendations Date Scrip I-Direct Code Action Initiation Range Target Stoploss Duration 29-Sep-21 SJVN SJVLIM Buy 28.3-29 31.50 27.00 14 Days 29-Sep-21 National Aluminium NATALU Buy 92-94 101.00 86.50 07 Days Intraday recommendations are for current month futures. Positional recommendations are in cash segment Retail Equity Research Retail – October 1, 2021 For Instant stock ideas: SUBSCRIBE to mobile notification on ICICIdirect Mobile app… Research Analysts Securities ICICI Dharmesh Shah Nitin Kunte, CMT Ninad Tamhanekar, CMT [email protected] [email protected] [email protected] Pabitro Mukherjee Vinayak Parmar [email protected] [email protected] NSE (Nifty): 17618 Technical Outlook NSE Nifty Daily Candlestick Chart Domestic Indices Day that was… Open High Low Close Indices Close 1 Day Chg % Chg Equity benchmarks concluded the monthly expiry session on a subdued note tracking mixed global cues. -

NSE Symbol NSE 6 Month Avg Total Market
Average Market Cap of 200 listed companies on BSE & NSE for the six months ended 30 June 2021 BSE 6 month Avg NSE 6 month Avg Average of BSE and NSE 6 Total Market Cap Total Market Cap month Avg Total Market Cap S.No. Company Name ISIN BSE SYMBOL (Rs. In Crs.) NSE Symbol (Rs. In Crs.) (Rs. in Crs.) 1 Reliance Industries Ltd INE002A01018 RELIANCE 1338017.01 RELIANCE 1355067.509 1346542.26 Tata Consultancy Services 2 Ltd. INE467B01029 TCS 1169783.56 TCS 1173068.166 1171425.86 3 HDFC Bank Ltd. INE040A01034 HDFCBANK 819037.95 HDFCBANK 818713.671 818875.81 4 Infosys Ltd INE009A01021 INFY 579784.19 INFY 579697.3885 579740.79 5 Hindustan Unilever Ltd., INE030A01027 HINDUNILVR 549336.78 HINDUNILVR 549358.908 549347.84 Housing Development 6 Finance Corp.Lt INE001A01036 HDFC 462288.58 HDFC 461373.1089 461830.84 7 ICICI Bank Ltd. INE090A01021 ICICIBANK 416645.51 ICICIBANK 416389.0234 416517.27 8 Kotak Mahindra Bank Ltd. INE237A01028 KOTAKBANK 361640.52 KOTAKBANK 361438.6361 361539.58 9 State Bank Of India, INE062A01020 SBIN 329767.32 SBIN 329789.268 329778.29 10 Bajaj Finance Limited INE296A01024 BAJFINANCE 324996.53 BAJFINANCE 324843.5005 324920.02 11 Bharti Airtel Ltd. INE397D01024 BHARTIARTL 299981.36 BHARTIARTL 299955.7729 299968.57 12 HCL Technologies Ltd INE860A01027 HCLTECH 261400.46 HCLTECH 261392.0109 261396.24 13 Wipro Ltd., INE075A01022 WIPRO 258617.45 WIPRO 261102.3994 259859.92 14 ITC Ltd INE154A01025 ITC 259423.16 ITC 259396.0648 259409.61 15 Asian Paints Ltd. INE021A01026 ASIANPAINT 253487.28 ASIANPAINT 253454.4536 253470.87 16 AXIS Bank Ltd.