Haldane's Rule and American Black Duck × Mallard Hybridization
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2019 Waterfowl Population Status Survey
U.S. Fish & Wildlife Service Waterfowl Population Status, 2019 Waterfowl Population Status, 2019 August 19, 2019 In the United States the process of establishing hunting regulations for waterfowl is conducted annually. This process involves a number of scheduled meetings in which information regarding the status of waterfowl is presented to individuals within the agencies responsible for setting hunting regulations. In addition, the proposed regulations are published in the Federal Register to allow public comment. This report includes the most current breeding population and production information available for waterfowl in North America and is a result of cooperative eforts by the U.S. Fish and Wildlife Service (USFWS), the Canadian Wildlife Service (CWS), various state and provincial conservation agencies, and private conservation organizations. In addition to providing current information on the status of populations, this report is intended to aid the development of waterfowl harvest regulations in the United States for the 2020–2021 hunting season. i Acknowledgments Waterfowl Population and Habitat Information: The information contained in this report is the result of the eforts of numerous individuals and organizations. Principal contributors include the Canadian Wildlife Service, U.S. Fish and Wildlife Service, state wildlife conservation agencies, provincial conservation agencies from Canada, and Direcci´on General de Conservaci´on Ecol´ogica de los Recursos Naturales, Mexico. In addition, several conservation organizations, other state and federal agencies, universities, and private individuals provided information or cooperated in survey activities. Appendix A.1 provides a list of individuals responsible for the collection and compilation of data for the “Status of Ducks” section of this report. -

Alpha Codes for 2168 Bird Species (And 113 Non-Species Taxa) in Accordance with the 62Nd AOU Supplement (2021), Sorted Taxonomically
Four-letter (English Name) and Six-letter (Scientific Name) Alpha Codes for 2168 Bird Species (and 113 Non-Species Taxa) in accordance with the 62nd AOU Supplement (2021), sorted taxonomically Prepared by Peter Pyle and David F. DeSante The Institute for Bird Populations www.birdpop.org ENGLISH NAME 4-LETTER CODE SCIENTIFIC NAME 6-LETTER CODE Highland Tinamou HITI Nothocercus bonapartei NOTBON Great Tinamou GRTI Tinamus major TINMAJ Little Tinamou LITI Crypturellus soui CRYSOU Thicket Tinamou THTI Crypturellus cinnamomeus CRYCIN Slaty-breasted Tinamou SBTI Crypturellus boucardi CRYBOU Choco Tinamou CHTI Crypturellus kerriae CRYKER White-faced Whistling-Duck WFWD Dendrocygna viduata DENVID Black-bellied Whistling-Duck BBWD Dendrocygna autumnalis DENAUT West Indian Whistling-Duck WIWD Dendrocygna arborea DENARB Fulvous Whistling-Duck FUWD Dendrocygna bicolor DENBIC Emperor Goose EMGO Anser canagicus ANSCAN Snow Goose SNGO Anser caerulescens ANSCAE + Lesser Snow Goose White-morph LSGW Anser caerulescens caerulescens ANSCCA + Lesser Snow Goose Intermediate-morph LSGI Anser caerulescens caerulescens ANSCCA + Lesser Snow Goose Blue-morph LSGB Anser caerulescens caerulescens ANSCCA + Greater Snow Goose White-morph GSGW Anser caerulescens atlantica ANSCAT + Greater Snow Goose Intermediate-morph GSGI Anser caerulescens atlantica ANSCAT + Greater Snow Goose Blue-morph GSGB Anser caerulescens atlantica ANSCAT + Snow X Ross's Goose Hybrid SRGH Anser caerulescens x rossii ANSCAR + Snow/Ross's Goose SRGO Anser caerulescens/rossii ANSCRO Ross's Goose -

The Regions of Maine MAINE the Maine Beaches Long Sand Beaches and the Most Forested State in America Amusements
the Regions of Maine MAINE The Maine Beaches Long sand beaches and The most forested state in America amusements. Notable birds: Piping Plover, Least Tern, also has one of the longest Harlequin Duck, and Upland coastlines and hundreds of Sandpiper. Aroostook County lakes and mountains. Greater Portland The birds like the variety. and Casco Bay Home of Maine’s largest city So will you. and Scarborough Marsh. Notable birds: Roseate Tern and Sharp-tailed Sparrow. Midcoast Region Extraordinary state parks, islands, and sailing. Notable birds: Atlantic Puffin and Roseate Tern. Downeast and Acadia Land of Acadia National Park, national wildlife refuges and state parks. Notable birds: Atlantic Puffin, Razorbill, and The Maine Highlands Spruce Grouse. Maine Lakes and Mountains Ski country, waterfalls, scenic nature and solitude. Notable birds: Common Loon, Kennebec & Philadelphia Vireo, and Moose River Downeast Boreal Chickadee. Valleys and Acadia Maine Lakes Kennebec & and Mountains Moose River Valleys Great hiking, white-water rafting and the Old Canada Road scenic byway. Notable birds: Warbler, Gray Jay, Crossbill, and Bicknell’s Thrush. The Maine Highlands Site of Moosehead Lake and Midcoast Mt. Katahdin in Baxter State Region Park. Notable birds: Spruce Grouse, and Black-backed Woodpecker. Greater Portland and Casco Bay w. e. Aroostook County Rich Acadian culture, expansive agriculture and A rich landscape and s. rivers. Notable birds: Three- cultural heritage forged The Maine Beaches toed Woodpecker, Pine by the forces of nature. Grossbeak, and Crossbill. 0 5 10 15 20 25 30 Scale of Miles Contents maine Woodpecker, Yellow-bellied Flycatcher, Philadelphia Vireo, Gray Jay, Boreal Chickadee, Bicknell’s Thrush, and a variety of warblers. -

American Black Duck Facts
U.S. Fish & Wildlife Service American Black Duck Facts Identification Male and female black ducks are similar to female mallards in appearance, but black ducks are darker overall, and have a dark tail. The male has a greenish-yellow bill, and the female’s bill is dark olive. In flight, they have a purple wing-patch on the upper-wing with no white edges. Their silvery-white under-wing linings contrast sharply with these ducks’ dark © Lang Elliot bodies. American Black Duck Life History American black ducks breed in a variety of wetland habitats, from salt marshes to beaver ponds, river islands, and boreal bogs. They winter in salt water along the coasts, but also in a variety of freshwater areas inland, like Cayuga Lake. Black ducks are dabbling ducks, tipping their bodies—head down, tail up— into shallow water to feed. They eat seeds, roots, stems, grain, aquatic © Doug Racine plants and insects, crustaceans, mollusks, and Hen Mallard Duck some small fish. Black duck nests are built on dirt mounds, hidden by vegetation, made of grass and weed stems, and lined with down. After incubation of the eggs, the male black duck does not participate in nesting. Female black ducks lay about 9 eggs, with a range of 7 to 12, and incubates them for 26 to 29 days. Nestlings leave the nest within hours of hatching and remain with their mother until they can fly, at about 60 days. Black Duck X Mallard Hybrid U.S. Fish & Wildlife Service American Black Duck Facts Migration and Wintering Black ducks are most common in the Atlantic and Mississippi flyways, with more numbers along the Atlantic coast. -

An Evaluation of Captive-Reared Mallard Releases in Virginia
AN EVALUATION OF CAPTIVE-REARED MALLARD RELEASES IN VIRGINIA Prepared by DGIF Mallard Release Committee July 2007 Table of Contents I. EXECUTIVE SUMMARY II. INTRODUCTION AND BACKGROUND III. CONCERNS REGARDING CAPTIVE-REARED MALLARD RELEASES A. Diseases Avian Influenza Duck Viral Enteritis Botulism Avian Cholera B. GENETIC DIVERSITY AND HYBRIDIZATION C. CONFOUNDING WATERFOWL MANAGEMENT PROGRAMS Aerial Waterfowl Surveys Waterfowl Breeding Population Surveys Banding Programs Harvest Surveys D. LAW ENFORCEMENT ISSUES E. ADMINISTRATIVE ISSUES F. ECOLOGICAL AND SOCIAL CONCERNS Nuisance issues Impact to waterfowl habitat IV. Illegal Releases V. DEFINITIONS, LAWS AND REGULATIONS PERTAINING TO THE RELEASE OF CAPTIVE- REARED MALLARDS VI. CONCLUSION APPENDIX A GEOGRAPHIC LOCATIONS OF MRA IN VIRGINIA AN EVALUATION OF CAPTIVE-REARED MALLARD RELEASES IN VIRGINIA I. EXECUTIVE SUMMARY This paper is a review of the practice of releasing captive-reared mallards on shooting preserves in Virginia and will be used by the Department of Game & Inland Fisheries (VDGIF) in evaluating this activity. This review details the potential problems associated with the release of captive-reared mallards. The issues have been well documented and include: disease risks to wild waterfowl and the domestic poultry industry, genetic mixing with wild mallard ducks and hybridization with black ducks, difficulties in surveying and assessing wild duck populations for management and regulatory purposes, ambiguities in wildlife law enforcement concerning live decoys and baiting regulations, and effects on habitats and the ecological community. These factors, along with the costs of controlling disease outbreaks and nuisance duck problems, far outweigh the short-term benefits that mallard release programs offer to a very small percentage of Virginia’s waterfowl hunters. -

Upland Game Birds and Waterfowl
OREGON DEPARTMENT OF FISH AND WILDLIFE 3406 CHERRY AVENUE, NE, SALEM, OR 97303 WILDLIFE DIVISION TELEPHONE: (503) 947-6300 GAME BIRD PROGRAM RECOMMENDATIONS FOR 2009-2010 GAME BIRD SEASONS – UPLAND GAME BIRDS AND WATERFOWL SUPPLEMENTAL RECOMMENDATIONS FOR MIGRATORY GAME BIRD SEASONS FOR CONSIDERATION BY THE OREGON FISH AND WILDLIFE COMMISSION AUGUST 7, 2009 TABLE OF CONTENTS Topic Page Introduction 3 Migratory Game Birds 4 Pacific Flyway Populations 4 Harvest Surveys 5 Migratory Game Bird Season Proposals 6 Duck and Merganser 6 General Fall Goose 8 NW Oregon Permit Goose 12 Black Brant 18 Wilson’s Snipe 18 American Coot 19 Falconry 20 Public Hunting Opportunities 21 Columbia Basin Regulated Hunt Areas 21 Appendix A – Adaptive Harvest Management Report, 2009 Appendix B – USFWS Waterfowl Population Status, 2009 Appendix C – Public Correspondence The recommendations in this packet are based on public correspondence (including telephone and e-mail communications), Pacific Flyway Study Committee and Council discussions, discussions with ODFW field personnel, federal regulatory requirements and past Oregon Fish and Wildlife Commission direction concerning hunting seasons. 2 INTRODUCTION This is a supplemental package provided to the Oregon Fish and Wildlife Commission (Commission), which outlines recommendations for most waterfowl seasons. The lateness of the federal regulatory process in 2009 precluded developing many season recommendations until this time. Season recommendations for upland game birds and some migratory game birds were included in an earlier Commission packet and are not repeated here. New or updated information on population status and harvest surveys is included. Detailed information on the status of waterfowl and Adaptive Harvest Management, including the western mallard model, is provided in two reports provided in the appendices. -
![American Black Duck Anas Rubripes [B,W]](https://docslib.b-cdn.net/cover/6370/american-black-duck-anas-rubripes-b-w-2176370.webp)
American Black Duck Anas Rubripes [B,W]
Appendix A: Birds American Black Duck Anas rubripes [B,W] Federal Listing N/A State Listing SGCN Global Rank G5 State Rank S4 Regional Status Very High Photo by Pamela Hunt Justification (Reason for Concern in NH) In the past 20 years, mid‐winter black duck surveys indicated that populations were declining from 1995‐2005, but have somewhat stabilized during 2006‐2015. Wintering black duck numbers have declined dramatically both in total and in the Atlantic Flyway from population numbers observed in the 1950s (USFWS 2015). The American Black Duck was ranked as the highest conservation concern (HH) for both Bird Conservation Regions (BCR) 14 and 30 and ranked high Regional priority (rank = 3). The black duck is the most important harvested duck in Canada and is considered a trophy species in the United States. The black duck was once the most common duck in New Hampshire (Lacaillade 1975), though since 2001 is has been only the third most abundant puddle duck harvested (NHFG duck harvest unpublished data). Distribution Black ducks primary breeding range is in Eastern Canada, the Maritime Provinces, and south to northern New England. Wintering populations are found primarily along the Atlantic Coast from New England south to the Carolinas and inland locations extending to the Mississippi River and its tributaries (Baldassarre, G. A. 2014). In New Hampshire, black ducks are found throughout the state and are the third most common duck species harvested (Northeast Breeding Plot Survey 2015, unpublished data). Black ducks winter primarily in coastal salt marshes and on Great Bay and are the most common winter dabbling duck in coastal marshes (MWS 2015, unpublished data). -
Valentine National Wildlife Refuge: Wildlife List
U.S. Fish & Wildlife Service Valentine National Wildlife Refuge Wildlife List Wildlife Abounds Valentine National Wildlife Refuge Hackberry and Look for ducks and geese, especially in the Native (NWR), located 25 miles south of Pelican Lakes during the spring and fall. Watch for Prairie the town of Valentine, Nebraska, is pintail, mallard, ruddy, canvasback, 71,774 acres in size and was established and many more ducks. Take a walk in 1935 as a Refuge and breeding on the nature trail up to the old fire grounds for migratory birds and tower on the west end of Hackberry other wildlife. In fact, most of the Lake for a view of the Sandhills and wildlife present in historical times a look at grassland sparrows. This goose, are still present on the Refuge designed by J.N. today. Numerous wetlands, lakes, Duck Lake Look in the trees around the boat “Ding” Darling, wet meadows, and large expanses of ramp for they are an oasis for has become the native prairie attract a wide variety songbirds. Watch for warblers, blue symbol of the of wildlife. This brochure lists and black-headed grosbeaks, Lazuli National Wildlife 289 species of birds, 41 species of buntings, eastern bluebirds, and Refuge System. mammals, 16 species of reptiles, and many more. six species of amphibians that have been recorded on the Refuge. Check-list Key Sp Spring March – May S Summer June – August May, September, and October offer F Fall September – November good opportunities for observing a W Winter December – February variety of migratory birds. Spring migrants, including waterfowl and c common – present in large warblers, are most numerous in May. -

Black Duck Outcome Management Strategy 2015–2025, V.1
Black Duck Outcome Management Strategy 2015–2025, v.1 I. Introduction The American black duck has been called the “gold standard” of eastern waterfowl. Historically, the black duck was the most abundant dabbling duck in eastern North America and comprised the largest portion of the region’s waterfowl harvest. Despite its importance to hunters and outdoor enthusiasts, the continental black duck population declined by more than 50 percent between the 1950s and 80s. Scientists believe this is due to loss of food and habitat associated with changing land use. The mid- Atlantic region, which includes the Chesapeake Bay watershed, supports the largest portion of eastern North America’s wintering black duck population, and preserving habitat here is critical to the long-term sustainability of the species. Black ducks are subjected to a variety of stressors during their annual lifecycle, many of which are beyond control of managers in the watershed. However, managers strive to provide enough food for ducks using the Atlantic Flyway during the winter months to support the Chesapeake’s historical proportion of the continental population goal set by the North American Waterfowl Management Plan (NAWMP). As an important indicator species, restoration of habitat for black ducks will also benefit other waterfowl which winter in the Bay region. 1 Chesapeake Bay Management Strategy Black Duck Outcome II. Goal, Outcome and Baseline This management strategy for the Chesapeake Bay Watershed Agreement identifies approaches for achieving the following goal and outcome: Vital Habitats Goal Restore, enhance and protect a network of land and water habitats to support fish and wildlife, and to afford other public benefits, including water quality, recreational uses and scenic value across the watershed. -
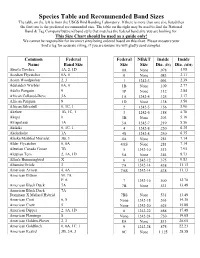
Species Table and Recommended Band Sizes the Table on the Left Is from the USGS Bird Banding Laboratory
Species Table and Recommended Band Sizes The table on the left is from the USGS Bird Banding Laboratory. If there is more than one size listed then the first one is the preferred recommended size. The table on the right may be used to find the National Band & Tag Company butt-end band style that matches the federal band size you are looking for. This Size Chart should be used as a guide only! We cannot be responsible for incorrect sizes being ordered based on this chart. Please measure your bird’s leg for accurate sizing, if you are unsure we will gladly send samples. Common Federal Federal NB&T Inside Inside Name Band Size Size Size Dia. (IN) Dia. (MM) Abert's Towhee 1A, 2, 1D 0A None .078 1.98 Acadian Flycatcher 0A, 0 0 None .083 2.11 Acorn Woodpecker 2, 3 1 1242-3 .094 2.39 Adelaide's Warbler 0A, 0 1B None .109 2.77 Adelie Penguin 9 1P None .112 2.84 African Collared-Dove 3A 1A 1242-4 .125 3.17 African Penguin 9 1D None .138 3.50 African Silverbill 0, 1C, 1 2 1242-5 .156 3.96 Akekee 1B, 1C, 1 3 1242-6 .188 4.78 Akepa 0 3B None .203 5.16 Akiapolaau 1A 3A 1242-7 .219 5.56 Akikiki 0, 1C, 1 4 1242-8 .250 6.35 Akohekohe 1A 4S 1242-8 .250 6.35 Alaska Marbled Murrelet 3B, 3 4A None .281 7.14 Alder Flycatcher 0, 0A 4AS None .281 7.14 Aleutian Canada Goose 7B 5 1242-10 .313 7.95 Aleutian Tern 2, 1A, 1D 5A None .344 8.73 Allen's Hummingbird X 6 1242-12 .375 9.53 Altamira Oriole 3 7A 1242-14 .438 11.13 American Avocet 4, 4A 7AS 1242-14 .438 11.13 American Bittern M: 7A F: 6 7 1242-16 .500 12.70 American Black Duck 7A 7B None .531 13.49 American -

American Black Duck EN
Introduction This duck • is very wary and among the most difficult of all ducks to deceive • was once the most abundant dabbling duck in eastern North America, but is now only half as numerous as it was in the 1950s • returns to the same marshes each fall, sometimes even starving rather than migrating farther south if those marshes are frozen Description The sooty-brown American Black Duck Anas rubripes is a common sight in ponds and marshes in eastern Canada. It is the only common duck in eastern North America in which the sexes are almost identical in appearance. Male and female American Black Ducks resemble the female Mallard in size and appearance. Their brown bodies are darker than the Mallard’s, however, and lack the Mallard’s whitish outer tail-feathers and prominent white wing bars. The American Black Duck’s head and neck are a lighter brown than its torso, and there is a beautiful purplish-blue patch, or speculum, on the wing. In flight, the American Black Duck is identifiable by the flash of its white underwings. The colours of the legs and bill may be used to determine the age and sex of American Black Ducks. These differences led to an earlier belief that there were two subspecies, a northern, red-legged race, and a southern "common" one. Data from bird banding, or tracking birds by placing numbered aluminum bands around their legs, has demonstrated conclusively that this is not the case. About five percent of the wild ducks that look like American Black Ducks in eastern North America (in some local areas the percentage may be much higher) are actually hybrids, the result of cross-breeding between blacks and Mallards in the wild. -
Migrationigration Flyways
CCOMMONOMMON DDUCKUCK SPECIESSPECIES OOFF TTHEHE GGREATREAT LLAKESAKES PINTAIL NORTHERN SHOVELER MALLARD AMERICAN WIGEON Th e pintail is a less common migrant Th e shoveler occasionally breeds in Th e mallard is the most common duck Th e American wigeon can be identifi ed through the Great Lakes region. Th e the Great Lakes region and is a fairly species in the Great Lakes region and by its green eye-patch and distinctive pintail primarily breeds in prairie Canada common spring and fall migrant. Th e across North America. Th eir large size, white cap that earned it the nickname and the U.S. and is easily identifi ed by its shoveler is named for its unique large, green heads and orange legs make “baldpate”. Wigeon are relatively long, slender neck and distinct, pointed wide bill that it uses to fi lter plankton mallards easy to recognize. Mallards can common Great Lakes migrants and are tail. from the surface water. be found almost everywhere in the Great unique among waterfowl because they Lakes. often graze on upland grasses and clovers. MMigrationigration Flyways Flyways show the major migration routes for waterfowl in the United States, Canada and Mexico. Flyway boundaries are not always sharply defi ned, and the routes 4 followed by migratory birds are numerous: no two species follow exactly the same RUDDY DUCK path from beginning to end. Th e four LESSER SCAUP Th e ruddy duck can be identifi ed by its primary North American fl yways are the Th e lesser scaup earned the nickname small size, white and black face markings 1 Pacifi c Flyway (1), the Central Flyway “bluebill” because of its distinctly and bright blue bill.