A Link Between Ph Homeostasis and Colistin Resistance in Bacteria Pradip R
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2014 Annual Report
2014 Annual Report www.joinpdx.org 503.232.7052 Dear Friend & Supporter, Letter Relationship and community…. from the Director these are words you will hear often at JOIN. I have had the privilege of being a part of the JOIN communi- ty for 7 years—fi rst as a community partner, then as a Board member, later a staff member, and now as the 2015 Board of Directors Executive Director. Chris Bonner, President There is one story that brings home the point of what JOIN really Hasson Company means about building relationships and cultivating community. John and I fi rst met when I was working at JOIN’s Basic Service Margaret Bryant, Vice President Center, or what we call the “House.” He had been sleeping outside Bryant Garcia Benefi t Consultants for several years, coming to JOIN for basic needs like showers, a locker to store his belongings, and community. I would often fi nd Nathan BeaƩ y, Treasurer him in the House playing dominoes, or using one of our computers Umpqua Bank to check apartment availability or connect with his family on face- book. Anna Plumb, Secretary Multnomah County John’s life has been fi lled with struggles and what he calls “bad choices.” He has a history of addiction and many encounters with Fineke Brasser law enforcement. He has children and grand-children he hasn’t Community Volunteer seen in person for years. John also has a college education, a his- tory of well-paying and high power jobs, and beautiful stories of Russ Campbell friendships and family events. -

1959 Surname
Surname Given Age Date Page Maiden Note Abercrombie James W. 36 10-Jul B-3 Abercrombie Josephine 82 27-Nov B-3 Abraham Clarence F. 64 17-Jul B-3 Absher Donna 87 18-Dec A-14 Adam Louis S. 66 21-Jan B-3 Adamczyk Klemenz 86 6-Mar B-8 Adams Elizabeth 83 10-Dec B-3 Adams Elmer L. 19 14-May A-1 Adams Myrtle M. 78 9-Mar 11 Adams Richard Dale Indant 26-Feb B-3 Adams Robert D. 62 14-Jul A-11 Ader Jeanette L. 27 29-Mar B-3 Adler Vickie Lynn 17 months 1-Jul B-3 Adley Pearl Katherine 79 9-Jul A-11 Adrien Andrew 66 29-Nov B-3 Ahlborn Bertha M. 77 8-Oct B-3 Ahlgren Oscar F. 69 23-Apr B-3 Aistgem Dawn Marie Infant 25-May A-13 Albrecht Chester 61 12-Feb B-3 Aldridge William C. (Blackie) 72 23-Mar 11 Alexander Helen H. 54 29-Sep A-11 Alford Howard E. 63 19-Apr B-3 Alger L. Nora 91 18-Jun B-3 Allen Charles J. 52 11-Mar B-3 Allison Daisy 71 21-May B-3 Allshouse Marcella Wickersham 6-Nov B-3 Ally Susan Jane 6 29-May A-1 Amanta Sister Mary 58 28-Jun B-4 Ambler Infant Son Infant 30-Aug B-3 Amey William A. 81 19-Jan 11 Amhurst Pearl J. 48 8-Apr B-3 Andersen Cordelia 79 6-Dec B-3 Anderson Bertie 72 24-May B-3 Anderson Byron 68 27-Feb B-3 Anderson Lily 68 10-Nov A-11 Anderson Roy G. -

Telephone Directory of Domestic Relations Division
TELEPHONE DIRECTORY FOR DOMESTIC RELATIONS DIVISION Honorable Kevin M. Dougherty, Administrative Judge, Family Division Honorable Margaret T. Murphy, Supervising Judge, Domestic Relations Division Mary Lou Baker, Deputy Court Administrator, Domestic Relations Division ADMINISTRATION 1 ADMINISTRATIVE SERVICES 2 BENCH WARRANT UNIT 3 CASE CLOSURE UNIT 4 CLERK OF THE COURT 5 COURT SUPPORT UNIT 6 COURTROOMS 7 CUSTOMER SERVICE CENTER 8-9-10 DATA MANAGEMENT & SCHEDULING UNIT 11 DISTRICT ATTORNEYS 12 DOMESTIC VIOLENCE UNIT 13 FINANCIAL SERVICES DEPARTMENT 14-15-16 (ACCOUNTING UNIT – UDC UNIT – CHARGE UNIT – EDC UNIT) FINANCIAL SERVICES –TACTICAL ENF –WAGE ATTACHMENT 17 GENETIC TESTING 18 HOME INVESTIGATORS 23 INTAKE UNIT 19 INTERSTATE – INTER-COUNTY UNIT 20 INTERPRETERS 12 MAILROOM 21 MASTERS - CUSTODY 22-23 MASTERS - DIVORCE 23 MASTERS - SUPPORT 24 NETWORKING FOR JOBS 25 NURSERY 26 PARENT LOCATE SERVICE 27 QUALITY ASSURANCE TEAM 28 RECORD ROOM 29 SECURITY & SHERIFF=S DEPARTMENT 30 SIX-FORTY-THREE LAB 31 SUPPORT COMPLIANCE UNIT 32 SUPPORT ESTABLISHMENT UNIT (Supervisors/Conference Officers) 33-34 SUPPORT ESTABLISHMENT UNIT (Clerical Staff) 35 TACTICAL ENFORCEMENT (see Financial Page 17) TRAINING UNIT 36 TRIAL COMMISSIONER 37 WAGE ATTACHMENT UNIT 32 WRIT SERVERS UNIT (Special Projects) 38 TRAINING RESOURCE CENTER Rm 102 686-9188 Telephone Directory DR Rev 1-9-08 ADMINISTRATION OFFICE Fax Number 686-8858 Room Extension Honorable Kevin M. Dougherty (1801 Vine Street) 314 686-7970 Administrative Judge, Family Division (34 S. 11th Street) 304 686-7085 Honorable Margaret T. Murphy 209 686-7326 Supervising Judge, Domestic Relations Division Mary Lou Baker, Deputy Court Administrator 304 9306-9307 Domestic Relations Division Roy C. Chambers, III, Director 304 8355-9385 Building and Field Operations Joseph C. -

(Surname in Parentheses) = Bride's Maiden Name * = Colored LI = Date
LAST FIRST Current AGE POB LAST FIRST Current AGE POB Date of NAME NAME Residence NAME NAME Residence Marriage BRIDE BRIDE GROOM GROOM Aaron Mildred Irvington, NJ 26 PA Calabrese Edward Newark, NJ 25 NJ 20 May 1940 Elizabeth Abel Catherine Triangle, VA 18 VA Allen James Triangle, VA 23 VA 16 Dec 1939 Virginia Monroe Abel Dorothy Quantico, VA 18 VA Gass Edward Quantico, VA 28 MA 2 Sep 1939 Louise Abrams Miriam Ventnor City, 21 NJ Fertick Albert Philadelphia, 22 PA 29 Jul 1940 Frances NJ Abraham PA Abramson Sylvia Flushing, LI, 34 NY Tush Joseph Flushing, LI, 32 NY 23 Feb 1940 NY Sanford NY Accardi Anna Queens Village, 26 NY DiMaria Frank Brooklyn, NY 36 ITA 10 Oct 1940 (Aloi) NY Acha Myrtle Pontiac, MI 24 MI Ferguson John Nesbit Detroit, MI 35 PA 8 Apr 1939 Violet Rita Achter Sylvia Philadelphia, 21 NY Cohen Albert Philadelphia, 24 PA 29 Jul 1940 Beatrice PA PA Acker Edna Mae Philadelphia, 22 PA Tranausky Edward Philadelphia, 23 PA 11 May 1940 PA Martin PA Ackoff Anne Nancy Philadelphia, 22 PA Wallen Albert David Philadelphia, 35 PA 30 Jul 1940 PA PA Acty Sarah Washington, 21 VA Jones Bernard Glendale, MD 23 MD *5 Jul 1940 Elizabeth DC Summerfield POB = Place of Birth (Surname in parentheses) = Bride’s Maiden Name * = Colored LI = Date License Issued LAST FIRST Current AGE POB LAST FIRST Current AGE POB Date of NAME NAME Residence NAME NAME Residence Marriage BRIDE BRIDE GROOM GROOM Adair Mildred Jacksonville, 24 LA Hanlon Jack Harrisville, PA 24 PA 6 Jul 1939 FL Adair Margaret Washington, 42 DC Lane Franklin Washington, 44 CA 14 Oct 1939 -

MREB Cleared Protocols
MREB Cleared Protocols MREB# Date Cleared Title Faculty Faculty Dept Student Type Level of Project 2003 014 Feb-10-2003 I. Bourgeault Health Studies R. Walji Faculty 2004 064 May-11-2004 Canadian Professors as Public Intellectuals N. McLaughlin Sociology K. Turcotte Faculty 2004 157 Oct-24-2004 M. Cadieux Psychology Faculty 2005 061 Aug-10-2005 S. Mestelman Economics M. Rogers Faculty 2005 168 Mar-01-2006 An exploration of Self-Actualization (the mind-body- W. Shaffir Sociology Y. Le Blanc PhD energy connection) as a Holistic Health Practice: participant observation 2005 186 Feb-05-2006 B. Milliken Psychology Hannah Teja Faculty 2006 006 Feb-06-2006 Automaticity and Focus of Attention in Handwriting J. Starkes Kinesiology J. Cullen Faculty 2006 007 Feb-06-2006 Actively Building Capacity in Primary Care Research C. Levitt Family Medicine Faculty 2006 008 Feb-09-2006 Parents: The unacknowledged victims of childhood T. Vaillancourt Psychology J. Johnson PostDoc bullying 2006 009 Feb-09-2006 Community medicine focus groups I. Tyler Family Medicine M. Hau Medical Student 2006 011 Feb-21-2006 Code-switching and prosody in sentence comprehension C. Anderson Linguistics Faculty 2006 012 Feb-28-2006 Elicited Writing Errors K. Humphreys Psychology D. Pollock MA 2006 013 Apr-05-2006 Correlation Between Student's High-School Mathematics M. Lovric Mathematics M. Fioroni MA Backgrounds and Performance in First Year Mathematics at McMaster University 2006 014 Mar-08-2006 The Impossible Policy Problem? An Exploration of the V. Satzewich Sociology A. Walker MA Policy Roadblocks to Foreign Credential Recognition in Canada 2006 015 Feb-05-2006 Inclusive Occupational Therapy Education: A Pilot Survey B. -

Surname Given Age Date Page Maiden Note Aageberg Alice F. 101 11-Feb D-1 See Also Article Feb. 12, P. C-5 Abegg Frances J. 64 9
Surname Given Age Date Page Maiden Note Aageberg Alice F. 101 11-Feb D-1 See also article Feb. 12, p. C-5 Abegg Frances J. 64 9-Jun D-1 Abel Delores Ruth 57 9-Sep B-6 Abel Ralph 31-Dec D-5 Abell Thelma 80 18-Dec A-11 See article, p. A-11 Abney Raymond 83 15-Dec D-1 See article, p. D-1 Acheson Helen G. 61 26-Apr E-2 Adamczyk Richard J. 48 5-Nov C-10 Adamovich Michael 77 10-Feb D-1 Adams Altha B. 91 12-Jan C-2 Adams Corinne, Deputy 79 12-Mar C-3 See also article March 13, p. C-1 Adams Frances D. 53 10-Nov D-5 Malloy Adams Leonard Eugene 58 2-Jun D-3 (Preacher) Adams Marie Mae 75 10-Mar C-1 Adams Robert F. 72 11-Jan D-1 Adams Susan L. 35 3-Mar C-6 Adams Thomas A. 12 Februray 2 A-6 Adams Thomas P. 38 19-Jan A-8 Addison James Grover "J. G." 69 3-Apr C-1 Adelsperger Edward H. 69 25-Mar D-1 Adelsperger Helen 71 24-Nov B-5 Adkins Kathleen 70 3-Feb B-7 Adler Sophie 74 4-Feb D-1 Adolph Bernard 60 2-Oct C-2 Adzima Rose 26-May D-5 Kubeck Agee Hardy 66 13-Jan A-1 See article, p. A-1 Agerter Tim D. 22 19-May D-1 See also article, p. A-2 Ahearn Mary 82 22-Apr C-3 Doolin Akhtar Julie Wayman 26 15-Apr C-5 Alaimo Bartole (Bart) 66 24-Oct B-2 Alaimo Damiana 86 20-Jan C-5 Alarcon Damiana 89 26-Jun C-1 Albert Florence E. -

Chester County Marriages Grooms Index 1885-1930
Chester County Marriages Grooms Index 1885-1930 Groom's Last Name Groom's First Name Middle Name Groom's Date of Birth Groom's Age Bride's First Name Bride's Last Name Date of Application Date of Marriage Place of Marriage License # Mabin Daniel ThomasOctober 26, 1870 Ida Boyer December 29, 1902 Coatesville 9520 MacAdam Charles A 25 Bessie Kramer September 22, 1924 Phoenixville 25313 MacAdams Robert A 41 Mary Moses April 22, 1926 Kimberton 26349 MacAfee Jacob LApril 8, 1883 Annie Thomas May 7, 1904 Nantmeal Village 10390 MacAfee WalterMay 26, 1877 Carrie Eyrich July 13, 1901 Marsh 8479 MacAfee William P1863 Lizzie Murray July 31, 1886 307 MacArthur Hugh 22 Ruth Heller August 9, 1930 West Chester 30408 Macaulay John WDecember 20, 1872 Mary McClure December 10, 1902 Guthriesville 9469 MacCain Howard S 21 Ella Schroder April 17, 1930 West Chester 30051 MacCleary Howard Ellsworth 21 Evelyn Clarke January 8, 1927 West Chester 26948 MacDade William Henry 33 Nellie Wilson April 21, 1919 Phoenixville 21345 MacDaniel George 22 Gertrude Tallen June 29, 1927 Philadelphia 27332 MacDonald Frank CAugust 30, 1889 Alice Whibley April 15, 1911 Coatesville 15369 MacDonald Frederick T 45 Jane Chalfant February 8, 1916 Philadelphia 18984 MacDonald George 48 Elizabeth Ralph November 5, 1925 West Chester 26088 MacDonald Harry MOctober 13, 1873 Bertha Kerns June 18, 1913 West Grove 17110 MacDonald Samuel FJanuary 22, 1868 Carrie Mercer June 26, 1895 Coatesville 4855 MacDonald Stephen GJuly 5, 1872 Marie Vail September 28, 1899 Chatham 7164 MacDonald Walter -

Index to St. Louis, Missouri Naturalization Records Created After Sept
Index to St. Louis, Missouri Naturalization Records Created after Sept. 27, 1906 Alphabetical surname index L–M History & Genealogy Department St. Louis County Library 1640 S. Lindberg Blvd. St. Louis, Missouri 63131 314-994-3300, ext. 2070 [email protected] Index to St. Louis, Missouri Naturalization Records Created after Sept. 27, 1906 This index covers St. Louis, Missouri naturalization records created between October 1, 1906 and December 1928 and is based on the following sources: • Naturalizations, U.S. District Court—Eastern Division, Eastern Judicial District of Missouri, Vols. 1 – 82 • Naturalizations, U.S. Circuit Court— Eastern Division, Eastern Judicial District of Missouri, Vols. 5 – 21 Entries are listed alphabetically by surname, then by given name, and then numerically by volume number. Abbreviations and Notations SLCL = History and Genealogy Department microfilm number (St. Louis County Library) FHL = Family History Library microfilm number * = spelling taken from the signature which differed from name in index. How to obtain copies Photocopies of indexed articles may be requested by sending an email to the History and Genealogy Department at [email protected]. A limit of three searches per request applies. Please review the library's lookup policy at https://www.slcl.org/genealogy-and-local- history/services. A declaration of intention may lead to further records. For more information, contact the National Archives at the address below. Include all information listed on the declaration of intention. National Archives, Central Plains Region 400 W. Pershing Rd. Kansas City, MO 64108 (816) 268-8000 [email protected] History Genealogy Dept. Index to St. Louis, Missouri Naturalization Records St. -

2012 Commencement Program
Emory University The One Hundred Sixty-Seventh Commencement The Fourteenth of May Two Thousand Twelve The Alma Mater Table of Contents In the heart of dear old Emory Where the sun doth shine, Order of Exercises .................................................................... 2 That is where our hearts are turning ’Round old Emory’s shrine. Musical Selections .................................................................... 3 Order of Procession ................................................................. 3 We will ever sing thy praises, Award Recipients ..................................................................... 4 Sons and daughters true. Hail we now our Alma Mater, Honorary Degree Recipients .................................................... 6 Hail the Gold and Blue! Diploma Ceremonies ................................................................ 7 Retiring Faculty and Staff ........................................................ 8 Tho’ the years around us gather, Crowned with love and cheer, Recipients of Degrees-in-Course ............................................... 9 Still the memory of Old Emory Grows to us more dear. Emory College of Arts and Sciences ..................................... 9 Oxford College .................................................................. 14 We will ever sing thy praises, Sons and daughters true. School of Medicine ............................................................ 14 Hail we now our Alma Mater, Nell Hodgson Woodruff School of Nursing ...................... -

2013 Annual Report
2013 Annual Report JOIN’s mission is to support the eff orts of homeless individuals and families to transi on off the street and into permanent housing. Our vision is a community where homelessness, if it exists at all, is a short-term circumstance rather than a long-term or chronic condi on. Letter from the Executive Director Three mes a week for two hours a day, Darryl organizes and staff s the clothing closet in JOIN’s day center. Darryl was homeless for decades before working with a JOIN outreach worker to fi nally return to housing. His path off the street – like everyone’s – was uniquely his, and JOIN was there to support him. In support of our na onal eff ort to end homelessness, offi ces exist in mul- ple federal, state, and local bureaucracies staff ed by people who want to do the right thing for homeless people. Hundreds of non-profi ts work to implement the latest recognized “best prac ces.” But as necessary as these I volunteer at JOIN because I organiza ons may be, they have never been the source of the best ideas want to give back. If it weren’t for how to address homelessness. The best ideas and the most important for you guys I’d sƟ ll be out resources for helping people end their homelessness come from the people there. I’ve been inside 3 whole experiencing homelessness themselves. This is one of the founding premis- years now and it’s great. My es of JOIN and it has been borne out over and over again by our experience health has improved, my dog is and by the research. -
Surname Index to Find All Instances of Your Family Name, Search for Variants Caused by Poor Handwriting, Misinterpretation of Similar Letters Or Their Sounds
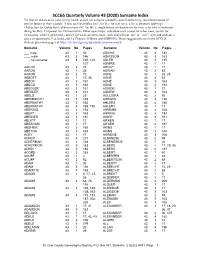
SCCGS Quarterly Volume 43 (2020) Surname Index To find all instances of your family name, search for variants caused by poor handwriting, misinterpretation of similar letters or their sounds. A few such examples are L for S, c for e, n for u, u for a; phonetic spellings (Aubuchon for Oubuchon); abbreviations (M’ for Mc ); single letters for double (m for mm, n for nn); translations (King for Roy, Carpenter for Zimmermann). Other search tips: substitute each vowel for other ones, search for nicknames, when hyphenated – search for each surname alone, with and without “de” or “von”; with and without a space or apostrophe (Lachance and La Chance, O’Brien and OBRIEN). More suggestions are on the SCCGS website Quarterly pages at https://stclair-ilgs.org/quarterly-surname-index/ Surname Volume No Pages Surname Volume No Pages ___ male 43 1 50 ADKINS 43 4 182 ___, male 43 3 146 ADKISSON 43 3 163 ___, no surname 43 3 120, 123, ADLER 43 3 135 127 ADMIRE 43 1 17 AACOX 43 2 74 ADOLF 43 1 17 AARON 43 1 25 ADRIAN 43 2 83 AARON 43 2 72 AGNE 43 1 24, 25 ABBOTT 43 1 17, 36 AGNE 43 2 62 ABECK 43 3 161 AGNE 43 3 163 ABEGG 43 3 163 AGNE 43 4 184 ABEGLER 43 3 161 AGNEW 43 1 31 ABEKLER 43 3 161 AGNEW 43 3 163 ABELS 43 1 25 AGULIERA 43 2 93 ABENDROTH 43 3 163 AHEARN 43 3 136 ABERNATHY 43 3 163 AHLERS 43 4 180 ABERNATHY 43 4 185, 190 AHLERT 43 1 17 ABERSOL 43 3 158 AHMANN 43 2 108 ABERT 43 3 163 AHRING 43 4 184 ABIGNER 43 3 161 AIGRE 43 3 161 ABLETT 43 1 17 AITKEN 43 1 17 ABRAHAM 43 1 17 AITKEN 43 4 197 ABSHIER 43 3 169 AKINS 43 1 17 ABSTON 43 3 163 AKINS 43 -
1960 to 1964 Volume 1: K-Q Obituary Index
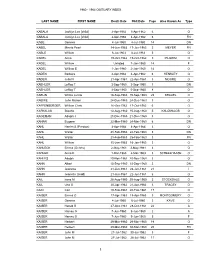
1960 - 1964 OBITUARY INDEX LAST NAME FIRST NAME Death Date Obit Date Page Also Known As Type KABALA Jacklyn Lee [child] 2-Apr-1962 3-Apr-1962 6 O KABALA Jacklyn Lee [child] 2-Apr-1962 4-Apr-1962 6 FN KABE Geneva 4-Jul-1960 4-Jul-1960 14 DN KABEL Minnie Pearl 14-Jun-1963 17-Jun-1963 5 MEYER FN KABLE William 6-Jul-1963 8-Jul-1963 5 O KADEL Anna 19-Oct-1962 19-Oct-1962 5 PILGRIM O KADEL William Unlisted 7-Jan-1960 14 F KADEL William E 1-Jan-1960 2-Jan-1960 14 O KADEN Barbara 2-Apr-1962 4-Apr-1962 6 HENSLEY O KADEN Julia N 21-Apr-1961 22-Apr-1961 3 MOORE O KAEHLER LeRoy T 3-Sep-1960 3-Sep-1960 7 DN KAEHLER LeRoy T 3-Sep-1960 5-Sep-1960 8 O KAELIN Wilma Lucille 18-Sep-1963 19-Sep-1963 25 KRAUEL O KAEMIE John Marion 24-Dec-1963 24-Dec-1963 3 O KAFFENBERGER William Chris 16-Oct-1962 17-Oct-1962 6 O KAFKALAS Staurla 13-Aug-1960 15-Aug-1960 5 KALOMALOS O KAGEMAN Adolph J 21-Dec-1963 21-Dec-1963 3 O KAHAN Eugene 22-Mar-1960 24-Mar-1960 6 DN KAHL Harriet S (Preston) 4-Apr-1964 4-Apr-1964 3 O KAHL Walter 21-Feb-1963 22-Feb-1963 5 DN KAHL Walter 21-Feb-1963 25-Feb-1963 3 FN KAHL William 15-Jan-1963 16-Jan-1963 3 O KAHLECK Emma (Gehrts) 2-May-1961 3-May-1961 4 O KAHLER Anna K 1-Mar-1963 2-Mar-1963 3 SCHEELHAASE O KAHLES Adolph 20-Nov-1964 20-Nov-1964 3 O KAHN Albert 13-Sep-1960 13-Sep-1960 5 DN KAHN Jeanette 21-Jun-1961 23-Jun-1961 21 I KAHN Jeanette (Gold) 21-Jun-1961 22-Jun-1961 6 O KAIL Irene M 28-Aug-1960 29-Aug-1960 5 STOCKDALE O KAIL Una O 20-Apr-1962 21-Apr-1962 3 TRACEY O KAIN Carl 19-Feb-1961 20-Feb-1961 17 O KAISER Emma O 17-Apr-1963 18-Apr-1963