Effects of Protected Areas and Climate Change on the Occupancy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Disaggregation of Bird Families Listed on Cms Appendix Ii
Convention on the Conservation of Migratory Species of Wild Animals 2nd Meeting of the Sessional Committee of the CMS Scientific Council (ScC-SC2) Bonn, Germany, 10 – 14 July 2017 UNEP/CMS/ScC-SC2/Inf.3 DISAGGREGATION OF BIRD FAMILIES LISTED ON CMS APPENDIX II (Prepared by the Appointed Councillors for Birds) Summary: The first meeting of the Sessional Committee of the Scientific Council identified the adoption of a new standard reference for avian taxonomy as an opportunity to disaggregate the higher-level taxa listed on Appendix II and to identify those that are considered to be migratory species and that have an unfavourable conservation status. The current paper presents an initial analysis of the higher-level disaggregation using the Handbook of the Birds of the World/BirdLife International Illustrated Checklist of the Birds of the World Volumes 1 and 2 taxonomy, and identifies the challenges in completing the analysis to identify all of the migratory species and the corresponding Range States. The document has been prepared by the COP Appointed Scientific Councilors for Birds. This is a supplementary paper to COP document UNEP/CMS/COP12/Doc.25.3 on Taxonomy and Nomenclature UNEP/CMS/ScC-Sc2/Inf.3 DISAGGREGATION OF BIRD FAMILIES LISTED ON CMS APPENDIX II 1. Through Resolution 11.19, the Conference of Parties adopted as the standard reference for bird taxonomy and nomenclature for Non-Passerine species the Handbook of the Birds of the World/BirdLife International Illustrated Checklist of the Birds of the World, Volume 1: Non-Passerines, by Josep del Hoyo and Nigel J. Collar (2014); 2. -

Malawi Trip Report 12Th to 28Th September 2014
Malawi Trip Report 12th to 28th September 2014 Bohm’s Bee-eater by Keith Valentine Trip Report compiled by Tour Leader: Keith Valentine RBT Malawi Trip Report September 2014 2 Top 10 Birds: 1. Scarlet-tufted Sunbird 2. Pel’s Fishing Owl 3. Lesser Seedcracker 4. Thyolo Alethe 5. White-winged Apalis 6. Racket-tailed Roller 7. Blue Swallow 8. Bohm’s Flycatcher 9. Babbling Starling 10. Bohm’s Bee-eater/Yellow-throated Apalis Top 5 Mammals: 1. African Civet 2. Four-toed Elephant Shrew 3. Sable Antelope 4. Bush Pig 5. Side-striped Jackal/Greater Galago/Roan Antelope/Blotched Genet Trip Summary This was our first ever fully comprehensive tour to Malawi and was quite simply a fantastic experience in all respects. For starters, many of the accommodations are of excellent quality and are also situated in prime birding locations with a large number of the area’s major birding targets found in close proximity. The food is generally very good and the stores and lodges are for the most part stocked with decent beer and a fair selection of South African wine. However, it is the habitat diversity that is largely what makes Malawi so good from a birding point of view. Even though it is a small country, this good variety of habitat, and infrastructure that allows access to these key zones, insures that the list of specials is long and attractive. Our tour was extremely successful in locating the vast majority of the region’s most wanted birds and highlights included Red-winged Francolin, White-backed Night Heron, African Cuckoo-Hawk, Western Banded Snake -

South Africa: Magoebaskloof and Kruger National Park Custom Tour Trip Report
SOUTH AFRICA: MAGOEBASKLOOF AND KRUGER NATIONAL PARK CUSTOM TOUR TRIP REPORT 24 February – 2 March 2019 By Jason Boyce This Verreaux’s Eagle-Owl showed nicely one late afternoon, puffing up his throat and neck when calling www.birdingecotours.com [email protected] 2 | TRIP REPORT South Africa: Magoebaskloof and Kruger National Park February 2019 Overview It’s common knowledge that South Africa has very much to offer as a birding destination, and the memory of this trip echoes those sentiments. With an itinerary set in one of South Africa’s premier birding provinces, the Limpopo Province, we were getting ready for a birding extravaganza. The forests of Magoebaskloof would be our first stop, spending a day and a half in the area and targeting forest special after forest special as well as tricky range-restricted species such as Short-clawed Lark and Gurney’s Sugarbird. Afterwards we would descend the eastern escarpment and head into Kruger National Park, where we would make our way to the northern sections. These included Punda Maria, Pafuri, and the Makuleke Concession – a mouthwatering birding itinerary that was sure to deliver. A pair of Woodland Kingfishers in the fever tree forest along the Limpopo River Detailed Report Day 1, 24th February 2019 – Transfer to Magoebaskloof We set out from Johannesburg after breakfast on a clear Sunday morning. The drive to Polokwane took us just over three hours. A number of birds along the way started our trip list; these included Hadada Ibis, Yellow-billed Kite, Southern Black Flycatcher, Village Weaver, and a few brilliant European Bee-eaters. -

Engelsk Register
Danske navne på alverdens FUGLE ENGELSK REGISTER 1 Bearbejdning af paginering og sortering af registret er foretaget ved hjælp af Microsoft Excel, hvor det har været nødvendigt at indlede sidehenvisningerne med et bogstav og eventuelt 0 for siderne 1 til 99. Tallet efter bindestregen giver artens rækkefølge på siden. -

Protected Area Management Plan
Karkloof Nature Reserve KwaZulu-Natal South Africa Protected Area Management Plan Prepared by Ezemvelo KwaZulu-Natal Wildlife Protected Area Management Planning Unit with support from Dr Richard Lechmere-Oertel (Biodiversity Planning & Management) Citation Karkloof Nature Reserve: Management Plan. Version 1.0 (2011), Ezemvelo KZN Wildlife, Pietermaritzburg. Photos for cover page courtesy of Kevin McCann and Di Martin. AUTHORISATION This Protected Area Management Plan for Karkloof Nature Reserve is recommended by the Karkloof Nature Reserve Co-Management Committee, consisting of three members each from the Karkloof Landowners Association and Ezemvelo KZN Wildlife according to the Co-Management Agreement and the Nature Reserve Planning Committee: Karkloof Landowners Association Thomas Hancock Thomas Hancock Children’s Family Trust Ezemvelo KZN Wildlife Andy Blackmore Acting manager land use Planning Magda Goosen Protected Area Management Planner Irene Hatton Acting Coordinator Protected Area Management Planning Bill Howells Biodiversity Conservation Co-ordinator East uKhahlamba Athol Marchant Regional Ecologist East uKhahlamba Philemon Mahlaba District Conservation Officer Ann van Eyssen Ecotourism Coordinator West KARKLOOF N AT U RE RESERVE I PRO T EC T ED A REA MA N AG EMEN T PLA N KARKLOOF N AT U RE RESERVE II PRO T EC T ED A REA MA N AG EMEN T PLA N KARKLOOF N AT U RE RESERVE IV PRO T EC T ED A REA MA N AG EMEN T PLA N TABLE OF CONTENTS AUTHORISATION ............................................................................................................................................. -

South Africa Mega Birding III 5Th to 27Th October 2019 (23 Days) Trip Report
South Africa Mega Birding III 5th to 27th October 2019 (23 days) Trip Report The near-endemic Gorgeous Bushshrike by Daniel Keith Danckwerts Tour leader: Daniel Keith Danckwerts Trip Report – RBT South Africa – Mega Birding III 2019 2 Tour Summary South Africa supports the highest number of endemic species of any African country and is therefore of obvious appeal to birders. This South Africa mega tour covered virtually the entire country in little over a month – amounting to an estimated 10 000km – and targeted every single endemic and near-endemic species! We were successful in finding virtually all of the targets and some of our highlights included a pair of mythical Hottentot Buttonquails, the critically endangered Rudd’s Lark, both Cape, and Drakensburg Rockjumpers, Orange-breasted Sunbird, Pink-throated Twinspot, Southern Tchagra, the scarce Knysna Woodpecker, both Northern and Southern Black Korhaans, and Bush Blackcap. We additionally enjoyed better-than-ever sightings of the tricky Barratt’s Warbler, aptly named Gorgeous Bushshrike, Crested Guineafowl, and Eastern Nicator to just name a few. Any trip to South Africa would be incomplete without mammals and our tally of 60 species included such difficult animals as the Aardvark, Aardwolf, Southern African Hedgehog, Bat-eared Fox, Smith’s Red Rock Hare and both Sable and Roan Antelopes. This really was a trip like no other! ____________________________________________________________________________________ Tour in Detail Our first full day of the tour began with a short walk through the gardens of our quaint guesthouse in Johannesburg. Here we enjoyed sightings of the delightful Red-headed Finch, small numbers of Southern Red Bishops including several males that were busy moulting into their summer breeding plumage, the near-endemic Karoo Thrush, Cape White-eye, Grey-headed Gull, Hadada Ibis, Southern Masked Weaver, Speckled Mousebird, African Palm Swift and the Laughing, Ring-necked and Red-eyed Doves. -

Zimbabwe Zambia Malawi Species Checklist Africa Vegetation Map
ZIMBABWE ZAMBIA MALAWI SPECIES CHECKLIST AFRICA VEGETATION MAP BIOMES DeserT (Namib; Sahara; Danakil) Semi-deserT (Karoo; Sahel; Chalbi) Arid SAvannah (Kalahari; Masai Steppe; Ogaden) Grassland (Highveld; Abyssinian) SEYCHELLES Mediterranean SCruB / Fynbos East AFrican Coastal FOrest & SCruB DrY Woodland (including Mopane) Moist woodland (including Miombo) Tropical Rainforest (Congo Basin; upper Guinea) AFrO-Montane FOrest & Grassland (Drakensberg; Nyika; Albertine rift; Abyssinian Highlands) Granitic Indian Ocean IslandS (Seychelles) INTRODUCTION The idea of this booklet is to enable you, as a Wilderness guest, to keep a detailed record of the mammals, birds, reptiles and amphibians that you observe during your travels. It also serves as a compact record of your African journey for future reference that hopefully sparks interest in other wildlife spheres when you return home or when travelling elsewhere on our fragile planet. Although always exciting to see, especially for the first-time Africa visitor, once you move beyond the cliché of the ‘Big Five’ you will soon realise that our wilderness areas offer much more than certain flagship animal species. Africa’s large mammals are certainly a big attraction that one never tires of, but it’s often the smaller mammals, diverse birdlife and incredible reptiles that draw one back again and again for another unparalleled visit. Seeing a breeding herd of elephant for instance will always be special but there is a certain thrill in seeing a Lichtenstein’s hartebeest, cheetah or a Lilian’s lovebird – to name but a few. As a globally discerning traveller, look beyond the obvious, and challenge yourself to learn as much about all wildlife aspects and the ecosystems through which you will travel on your safari. -

Zambia and Malawi Trip Report – August/September 2014
Zambia and Malawi Trip Report – August/September 2014 Miombo Tit www.birdingecotours.com [email protected] 2 | T R I P R E P O R T Zambia and Malawi August/September 2014 This trip was run as a customized tour for three clients, all with lists of well over 7000 species seen worldwide, and in fact Dollyann was hoping to reach 8000 species by the end of this trip. Travel to some really remote destinations, particularly in Malawi, was necessary to find some of the group’s target birds. Places like Misuku Hills and Uzumara Forest in Malawi are hardly ever visited by birders, primarily from a logistics point of view, and also because of lack of suitable accommodation. Both these destinations are, however, excellent birding spots, and Uzumara in particular could be included in most itineraries, using accommodation in the town of Rumphi as a base. On the Zambian side we included the Mwinilunga area, a must for any serious birder; this area hosts many Angolan/Congo specials, found nowhere else in Zambia. Day 1, 14th August. Livingstone Airport to Lodge Ron, Dollyann, and Kay arrived on the same flight from Johannesburg at around 13h00. After a short meet and greet and a quick visit to the bank for some local currency, we loaded up and started our journey to our lodge. Not much was seen en route other than a few marauding Pied Crows and a single African Grey Hornbill. We arrived at the lodge in good time and decided to take 20 minutes to refresh, before starting our bird quest. -

Project Name
SYRAH RESOURCES GRAPHITE PROJECT, CABO DELGADO, MOZAMBIQUE TERRESTRIAL FAUNAL IMPACT ASSESSMENT Prepared by: Prepared for: Syrah Resources Limited Coastal and Environmental Services Mozambique, Limitada 356 Collins Street Rua da Frente de Libertação de Melbourne Moçambique, Nº 324 3000 Maputo- Moçambique Australia Tel: (+258) 21 243500 • Fax: (+258) 21 243550 Website: www.cesnet.co.za December 2013 Syrah Final Faunal Impact Assessment – December 2013 AUTHOR Bill Branch, Terrestrial Vertebrate Faunal Consultant Bill Branch obtained B.Sc. and Ph.D. degrees at Southampton University, UK. He was employed for 31 years as the herpetologist at the Port Elizabeth Museum, and now retired holds the honorary post of Curator Emeritus. He has published over 260 scientific articles, as well as numerous popular articles and books. The latter include the Red Data Book for endangered South African reptiles and amphibians (1988), and co-editing its most recent upgrade – the Atlas and Red Data Book of the Reptiles of South Africa, Lesotho and Swaziland (2013). He has also published guides to the reptiles of both Southern and Eastern Africa. He has chaired the IUCN SSC African Reptile Group. He has served as an Honorary Research Professor at the University of Witwatersrand (Johannesburg), and has recently been appointed as a Research Associate at the Nelson Mandela Metropolitan University, Port Elizabeth. His research concentrates on the taxonomy, biogeography and conservation of African reptiles, and he has described over 30 new species and many other higher taxa. He has extensive field work experience, having worked in over 16 African countries, including Gabon, Ivory Coast, DRC, Zambia, Mozambique, Malawi, Madagascar, Namibia, Angola and Tanzania. -

Lazy Cisticola Movements in Southern Africa (Irwin 1981; Tarboton Et Al
314 Sylviidae: warblers, apalises, crombecs, eremomelas, cisticolas and prinias during the breeding season. It is not known to show local Lazy Cisticola movements in southern Africa (Irwin 1981; Tarboton et al. Luitinktinkie 1987b; Johnson & Maclean 1994). Breeding: Atlas records were all during the wet summer Cisticola aberrans months, October–April. Egglaying has been recorded October–February in KwaZulu-Natal (Dean 1971), Octo- The Lazy Cisticola is widely distributed in southcentral ber–March in the Transvaal and Botswana (Tarboton et al. Africa and fairly common in the moist eastern and north- 1987b; Skinner 1995a), and September–April (mainly ern areas of southern Africa. Beyond southern Africa, it October–December) in Zimbabwe (Irwin 1981). occurs in Zambia, Malawi, Mozambique and Tanzania. In Interspecific relationships: It occupies similar habitats South Africa it is widespread from the eastern Cape Prov- to the Tawnyflanked Prinia and the two species appear to ince through KwaZulu-Natal, to the eastern Free State and have similar ecological requirements, although the Lazy the Transvaal. It is common in Swaziland and widely dis- Cisticola is more closely associated with rocky outcrops. tributed in Zimbabwe. It occurs sparsely in eastern Bot- The Lazy Cisticola uses lower vegetation levels than the swana. It does not occur above 1350 m in Zimbabwe (Irwin Rattling Cisticola C. chiniana. It may compete with the 1981) but is recorded up to 2440 m in the Drakensberg Wailing Cisticola, both species occurring on moist grassy range (Little & Bainbridge 1992). hillsides, and it is also thought to compete locally with the It occurs in pairs or family groups, and is unobtrusive, Redfaced Cisticola C. -
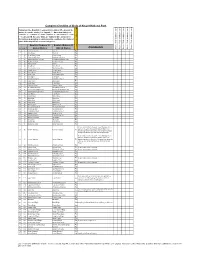
Kruger Comprehensive
Complete Checklist of birds of Kruger National Park Status key: R = Resident; S = present in summer; W = present in winter; E = erratic visitor; V = Vagrant; ? - Uncertain status; n = nomadic; c = common; f = fairly common; u = uncommon; r = rare; l = localised. NB. Because birds are highly mobile and prone to fluctuations depending on environmental conditions, the status of some birds may fall into several categories English (Roberts 7) English (Roberts 6) Comments Date of Trip and base camps Date of Trip and base camps Date of Trip and base camps Date of Trip and base camps Date of Trip and base camps # Rob # Global Names Old SA Names Rough Status of Bird in KNP 1 1 Common Ostrich Ostrich Ru 2 8 Little Grebe Dabchick Ru 3 49 Great White Pelican White Pelican Eu 4 50 Pinkbacked Pelican Pinkbacked Pelican Er 5 55 Whitebreasted Cormorant Whitebreasted Cormorant Ru 6 58 Reed Cormorant Reed Cormorant Rc 7 60 African Darter Darter Rc 8 62 Grey Heron Grey Heron Rc 9 63 Blackheaded Heron Blackheaded Heron Ru 10 64 Goliath Heron Goliath Heron Rf 11 65 Purple Heron Purple Heron Ru 12 66 Great Egret Great White Egret Rc 13 67 Little Egret Little Egret Rf 14 68 Yellowbilled Egret Yellowbilled Egret Er 15 69 Black Heron Black Egret Er 16 71 Cattle Egret Cattle Egret Ru 17 72 Squacco Heron Squacco Heron Ru 18 74 Greenbacked Heron Greenbacked Heron Rc 19 76 Blackcrowned Night-Heron Blackcrowned Night Heron Ru 20 77 Whitebacked Night-Heron Whitebacked Night Heron Ru 21 78 Little Bittern Little Bittern Eu 22 79 Dwarf Bittern Dwarf Bittern Sr 23 81 Hamerkop -

Ultimate Zambia (Including Pitta) Tour
BIRDING AFRICA THE AFRICA SPECIALISTS Ultimate Zambia including Zambia Pitta 2019 Tour Report © Yann Muzika © Yann African Pitta Text by tour leader Michael Mills Photos by tour participants Yann Muzika, John Clark and Roger Holmberg SUMMARY Our first Ultimate Zambia Tour was a resounding success. It was divided into three more manageable sections, namely the North-East Extension, © John Clark © John Main Zambia Tour and Zambia Pitta Tour, each with its own delights. Birding Africa Tour Report Tour Africa Birding On the North-East Pre-Tour we started off driving We commenced the Main Zambia Tour at the Report Tour Africa Birding north from Lusaka to the Bangweulu area, where spectacular Mutinondo Wilderness. It was apparent we found good numbers of Katanga Masked that the woodland and mushitu/gallery forest birds Weaver coming into breeding plumage. Further were finishing breeding, making it hard work to Rosy-throated Longclaw north at Lake Mweru we enjoyed excellent views of track down all the key targets, but we enjoyed good Zambian Yellow Warbler (split from Papyrus Yellow views of Bar-winged Weaver and Laura's Woodland Warbler) and more Katanga Masked Weavers not Warbler and found a pair of Bohm's Flycatchers finally connected with a pair of Whyte's Francolin, Miombo Tit, Bennett's Woodpecker, Amur Falcon, yet in breeding plumage. From here we headed east feeding young. Other highlights included African which we managed to flush. From Mutinondo Cuckoo Finch, African Scops Owl, White-crested to the Mbala area we then visited the Saisi River Barred Owlet, Miombo Rock Thrush and Spotted we headed west with our ultimate destination as Helmetshrike and African Spotted Creeper.