Otitis Media (Acute): Antimicrobial Prescribing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Perforated Eardrum
Vinod K. Anand, MD, FACS Nose and Sinus Clinic Perforated Eardrum A perforated eardrum is a hole or rupture m the eardrum, a thin membrane which separated the ear canal and the middle ear. The medical term for eardrum is tympanic membrane. The middle ear is connected to the nose by the eustachian tube. A perforated eardrum is often accompanied by decreased hearing and occasional discharge. Paih is usually not persistent. Causes of Eardrum Perforation The causes of perforated eardrum are usually from trauma or infection. A perforated eardrum can occur: if the ear is struck squarely with an open hand with a skull fracture after a sudden explosion if an object (such as a bobby pin, Q-tip, or stick) is pushed too far into the ear canal. as a result of hot slag (from welding) or acid entering the ear canal Middle ear infections may cause pain, hearing loss and spontaneous rupture (tear) of the eardrum resulting in a perforation. In this circumstance, there may be infected or bloody drainage from the ear. In medical terms, this is called otitis media with perforation. On rare occasions a small hole may remain in the eardrum after a previously placed P.E. tube (pressure equalizing) either falls out or is removed by the physician. Most eardrum perforations heal spontaneously within weeks after rupture, although some may take up to several months. During the healing process the ear must be protected from water and trauma. Those eardrum perforations which do not heal on their own may require surgery. Effects on Hearing from Perforated Eardrum Usually, the larger the perforation, the greater the loss of hearing. -

Otitis Media: Causes and Treatment
Otitis media: causes and treatment This leaflet is for patients with otitis media (infection of the middle ear). If you do not understand anything or have any other concerns, please speak to a member of staff. What is otitis media? It is inflammation and infection of the middle ear. This is the eardrum and the small space behind the eardrum. What causes otitis media? Inflammation and blockage of the Eustachian tube following chest infection, colds, flu and throat infection which can cause a build-up of mucus in the middle ear. What are the symptoms? • Earache. • Dulled hearing may develop for a few days. • Fever (high temperature). • Sometimes the eardrum perforates (bursts). This lets out infected mucus, and the ear becomes runny for a few days. As the pain is due to a tense eardrum, if the eardrum bursts, the pain often settles. A perforated eardrum usually heals quickly after the infection clears. It is important that during the next 6 weeks that the ear canal is kept dry during the healing process. Once the infection (and perforation) have cleared, your hearing should return to normal. What is the treatment for otitis media? Most bouts of ear infection will clear on their own within three days. The immune system can usually clear bacteria or viruses causing ear infections. • Painkillers such as Paracetamol or Ibuprofen will ease the pain and will also lower a raised temperature. It is important that you take painkillers as prescribed until the pain eases. • Antibiotics are prescribed if the infection is severe, or is getting worse after 2-3 days. -

Determinants of Conductive Hearing Loss in Tympanic Membrane Perforation
Clinical and Experimental Otorhinolaryngology Vol. 8, No. 2: 92-96, June 2015 http://dx.doi.org/10.3342/ceo.2015.8.2.92 pISSN 1976-8710 eISSN 2005-0720 Original Article Determinants of Conductive Hearing Loss in Tympanic Membrane Perforation Hanaro Park·Seung No Hong·Hyo Sang Kim·Jae Joon Han·Juyong Chung·Myung-Whan Seo·Seung-Ha Oh Sun-O Chang·Jun Ho Lee Department of Otorhinolaryngology-Head and Neck Surgery, Seoul National University College of Medicine, Seoul, Korea Objectives. Tympanic membrane perforations are common, but there have been few studies of the factors determining the extent of the resulting conductive hearing loss. The aims of this study were to determine whether the size of tympan- ic membrane perforation, pneumatization of middle ear & mastoid cavity, and location of perforation were correlated with air-bone gap (ABG) of patients. Methods. Forty-two patients who underwent tympanoplasty type I or myringoplasty were included and preoperative audi- ometry were analyzed. Digital image processing was applied in computed tomography for the estimation of middle ear & mastoid pneumatization volume and tympanic membrane photograph for the evaluation of perforation size and location. Results. Preoperative mean ABG increased with perforation size (P=0.018), and correlated inversely with the middle ear & mastoid volume (P=0.005). However, perforations in anterior versus posterior locations showed no significant dif- ferences in mean ABG (P=0.924). Conclusion. The degree of conductive hearing loss resulting from a tympanic membrane perforation would be expected with the size of perforation and pneumatization of middle ear and mastoid. Keywords. Tympanic Membrane Perforation; Tympanoplasty INTRODUCTION ear, and corresponding models have been suggested [1-3]. -

Onward Referral of Adults with Hearing Difficulty Directly Referred to Audiology Services
Guidance for Audiologists: Onward Referral of Adults with Hearing Difficulty Directly Referred to Audiology Services Produced by: Service Quality Committee of the British Academy of Audiology Key Authors: Hanna Jeffery Suzanne Jennings Laura Turton Date of publication: November 2016 (minor amendment July 2017) Review date: November 2021 BAA – Service Quality Committee Acknowledgements The Service Quality Committee would like to thank all those who provided their opinions on the draft of this document sent out for consultation, including BAA members, The British Society of Audiology, The British Association of Audiological Physicians, ENT UK and The Royal College of General Practitioners. This document is a British Academy of Audiology document and has not been endorsed by any other organisation. Introduction This document is intended to guide Audiologists in service planning and in making referrals for a medical or other professional opinion. Along with “Guidelines for Primary Care: Direct Referral of Adults with Hearing Difficulty to Audiology Services (2016)1”, this document replaces the earlier guidelines (BAA 20092, TTSA 19893,4) and has been approved by the Board of the British Academy of Audiology. This document comprises a set of criteria which define the circumstances in which an Audiologist in the UK should refer an adult with hearing difficulties for a medical or other professional opinion. If any of these are found, then the patient should be referred to an Ear, Nose and Throat (ENT) department, to their GP or to an Audiologist with an extended scope of practice. The criteria have been written for all adults (age 18+), but local specifications regarding age range for direct referral should be adhered to. -

NL0313A Hearing Loss – Introduction and Overview Printed with Permission from Better Hearing Institute
NL0313A Hearing Loss – Introduction and Overview Printed with Permission from Better Hearing Institute http://www.betterhearing.org The Better Hearing Institute (BHI) is a not-for-profit corporation that educates the public about the neglected problem of hearing loss and what can be done about it. Founded in 1973, we are working to: Erase the stigma and end the embarrassment that prevents millions of people from seeking help for hearing loss. Show the negative consequences of untreated hearing loss for millions of Americans. Promote treatment and demonstrate that this is a national problem that can be solved. 1. HOW WE HEAR Patricia E. Connelly, PhD, CCC-A, FAAA, New Jersey Medical School, NEWARK, NJ The Hearing System The anatomy of the hearing system can be divided into four components for our convenience in remembering the parts and associating these parts with their function. These divisions are the: 1. outer ear 2. middle ear 3. inner ear 4. central auditory pathways The Outer Ear (1) Several structures comprise the outer ear. The most readily seen is the pinna, also called the auricle. The pinna is made up of a frame of cartilage that is covered with skin. The pinna has obvious folds, elevations, depressions and a prominent bowl - all of which vary somewhat from person to person but a basic pattern in these features is fairly universal among all people. The pinna acts as a funnel to collect and direct sound down the ear canal. It also serves to enhance some sounds through its resonance characteristics. Finally, it helps us to appreciate front-back sound localization. -

Perforated Eardrum and Myringoplasty
Perforated eardrum and myringoplasty Disclaimer: The details in this section are for general information only. Always check with your own surgeon. What is a perforated eardrum? A perforated eardrum means there is a hole in the eardrum, which may have been caused by infection or injury. Quire often a hole in the eardrum may heal itself. Sometimes it does not cause any problems. However, it may cause recurrent infections with a discharge from the ear. If you have an infection you should avoid getting water in the ear. If the hole is large then you may experience some hearing loss. A hole in the eardrum can be identified by an ENT specialist using an instrument called an ‘auriscope’. Surgery to repair the perforation An operation to repair the perforation is called a ‘myringoplasty’. The benefits of closing a perforation include prevention of water entering the middle ear, which Source: Ear Nose & Throat Reference No: 6135-1 Issue date: 16/9/19 Review date: 16/9/22 Page 1 of 3 could cause ear infection. Repairing the hole means that you should get fewer ear infections. It may result in improved hearing, but repairing the eardrum alone seldom leads to great improvement in hearing. If the hole in the eardrum has only just occurred, no treatment may be required. You should discuss with your surgeon whether to wait and see, or have surgery now. You may change your mind about the operation at any time and signing a consent form does not mean that you have to have the operation. If you would like to have a second opinion about the treatment, you can ask your specialist. -
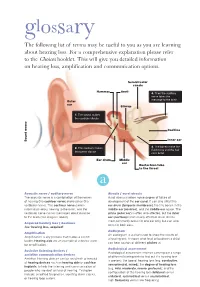
Glossary the Following List of Terms May Be Useful to You As You Are Learning About Hearing Loss
glossary The following list of terms may be useful to you as you are learning about hearing loss. For a comprehensive explanation please refer to the Choices booklet. This will give you detailed information on hearing loss, amplification and communication options. Semicircular canals Hammer Anvil 4. Then the auditory nerve takes the message to the brain. Outer ear Stirrup 1. The sound makes the eardrum vibrate Cochlea Inner ear Sound waves . The bones make the 2. The eardrum makes 3 fluid move and the hair the bones vibrate cells bend. Ear drum Middle ear Eustachian tube to the throat a Acoustic nerve / auditory nerve Atresia / aural atresia The acoustic nerve is a combination of the nerves Aural atresia involves some degree of failure of of hearing (the cochlear nerve) and balance (the development of the ear canal. It can also affect the vestibular nerve). The cochlear nerve carries ear drum (tympanic membrane), the tiny bones in the information about hearing to the brain, and the middle ear (ossicles), and the middle ear space. The vestibular nerve carries messages about balance pinna (outer ear) is often also affected, but the inner to the brain (see diagram above). ear (cochlea) is not usually affected. Aural atresia most commonly occurs in one ear only, but can also Acquired hearing loss / deafness occur in both ears. See ‘hearing loss, acquired’. Audiogram Amplification An audiogram is a chart used to show the results of Amplification is any process that makes a sound a hearing test. It shows what level of loudness a child louder. Hearing aids are an example of a device used can hear sounds of different pitches at. -

Different Types of Tinnitus – and What to Do About Them Different Types of Tinnitus – and What to Do About Them
Different types of tinnitus – and what to do about them Different types of tinnitus – and what to do about them This factsheet is part of our Tinnitus range. It is written for people who have tinnitus, their families, friends and the professionals who work with them. Tinnitus is a medical term to describe noises that people can hear in one ear, both ears or in the head – such as ringing, buzzing or whistling. The sounds heard can vary from person to person, but the common link is that they do not have an external source. This factsheet gives information about musical hallucinations, pulsatile tinnitus and low frequency noise. Read on to find out: What are musical hallucinations? Who experiences musical hallucinations? What causes musical hallucinations? How can I deal with musical hallucinations? What is pulsatile tinnitus? What causes pulsatile tinnitus? How is pulsatile tinnitus diagnosed? How can I deal with pulsatile tinnitus? What is low frequency noise? Why are some people more aware of low frequency noise than others? What are the effects of low frequency noise? How do I tell the difference between low frequency noise and tinnitus? How can I deal with low frequency noise? Where can I buy products that may help? Where can I get further information? If you would like this factsheet on audio tape, in Braille or in large print, please contact our helpline (see front page). Medical disclaimer: the information given in this factsheet is not medical advice and by providing it neither Action on Hearing Loss nor our tinnitus and medical advisors undertake any responsibility for your medical care, nor accept you as a patient. -

Interventions for Adult Eustachian Tube Dysfunction: a Systematic Review
HEALTH TECHNOLOGY ASSESSMENT VOLUME 18 ISSUE 46 JULY 2014 ISSN 1366-5278 Interventions for adult Eustachian tube dysfunction: a systematic review Alexis Llewellyn, Gill Norman, Melissa Harden, Andrew Coatesworth, Daniel Kimberling, Anne Schilder and Catriona McDaid DOI 10.3310/hta18460 Interventions for adult Eustachian tube dysfunction: a systematic review Alexis Llewellyn,1 Gill Norman,1 Melissa Harden,1 Andrew Coatesworth,2 Daniel Kimberling,3 Anne Schilder4 and Catriona McDaid1* 1Centre for Reviews and Dissemination, University of York, York, UK 2Ear, Nose and Throat Department, York Hospital, York, UK 3Gale Farm Surgery, York, UK 4evidENT University College London Ear Institute, Royal National Throat, Nose and Ear Hospital University College London, London, UK *Corresponding author Declared competing interests of authors: none Published July 2014 DOI: 10.3310/hta18460 This report should be referenced as follows: Llewellyn A, Norman G, Harden M, Coatesworth A, Kimberling D, Schilder A, et al. Interventions for adult Eustachian tube dysfunction: a systematic review. Health Technol Assess 2014;18(46). Health Technology Assessment is indexed and abstracted in Index Medicus/MEDLINE, Excerpta Medica/EMBASE, Science Citation Index Expanded (SciSearch®) and Current Contents®/ Clinical Medicine. Health Technology Assessment NICE TAR and DAR ISSN 1366-5278 (Print) ISSN 2046-4924 (Online) Five-year impact factor: 5.804 Health Technology Assessment is indexed in MEDLINE, CINAHL, EMBASE, The Cochrane Library and the ISI Science Citation Index and is assessed for inclusion in the Database of Abstracts of Reviews of Effects. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (www.publicationethics.org/). -

Repair of a Perforated Eardrum (Myringoplasty)
The Children’s Hospital Repair of a Perforated Eardrum (Myringoplasty) Information for parents and carers What is a perforated eardrum? A perforated eardrum means there is a hole in the eardrum. This may have been caused by infection or injury to the eardrum. Sometimes, a hole in the eardrum will heal itself. However a hole in the eardrum may cause recurrent infections with a discharge from the ear. If your child has an infection, they should avoid getting water in their ear. If the hole is large, your child may experience some hearing loss. A hole in the eardrum can be identified by a doctor or nurse specialist using an instrument called an auroscope. If the hole in the ear drum is causing discharge or deafness, your child’s surgeon may recommend that it is repaired. What is a myringoplasty? A myringoplasty is an operation to repair the perforation in the eardrum. The operation can successfully close a small hole nine times out of ten. The success rate is not as good if the hole is large. What are the benefits of the operation? The benefits include: • preventing water from entering the middle ear, which would cause ear infection • fewer ear infections • a possible improvement in hearing, but repairing the eardrum alone seldom leads to great improvement in hearing. An operation may be carried out on the ossicles (bones of hearing) at a later date, if necessary. page 2 Copyright © 2010 ENT•UK What are the risks? This is a simple and safe operation. However, all operations will carry some risks. -

A Modern Guide to Hearing Loss
A MODERN GUIDE www.mass.gov/mcdhh TO HEARING LOSS A MODERN NEW GUIDE EDITION 2019 TO HEARING For the Deaf and LOSS Hard of Hearing • FOR THE DEAF Massachusetts Commission for the AND Deaf and Hard of Hearing What’s Inside What’s New 600 Washington Street HARD • The Impact of Hearing Loss Understanding and Boston, MA 02111 • Where to Turn for Help Coping with Trauma OF • Emerging Technologies and Hearing Loss Phone Numbers: HEARING 617-740-1600 Voice 617-740-1700 TTY 617-740-1810 Fax EXCLUSIVE • Toll Free: 800-882-1155 Voice 2019 Toll Free: 800-530-7570 TTY Foreword includes personal stories from survivors VP 617-326-7546 of mass tragedies who suffered hearing loss Massachusetts Commission for the Deaf and Hard of Hearing A Modern Guide to Hearing Loss for the Deaf and Hard of Hearing Massachusetts Commission for the Deaf and Hard of Hearing Boston, MA All rights reserved by the Massachusetts Commission for the Deaf and Hard of Hearing. This book or any portion thereof may not be reproduced or used in any manner without the express written permission of the publisher. Funding by the Massachusetts Commission for the Deaf and Hard of Hearing (MCDHH) and the Massachusetts Office for Victim Assistance (MOVA) through the Antiterrorism Emergency Assis- tance Program, cooperative agreement number 2014-RF-GX-K002 from the Office for Victims of Crime, Office of the Justice Programs and the US Department of Justice. Copyright ©2019 Printed by FLAGSHIP PRESS, INC. 150 Flagship Drive • North Andover, MA Cover and book design by Denise Adkerson FOREWORD The Massachusetts Commission for the Deaf and Hard of Hearing is excited to have the opportunity to update its guide, originally known Tas the “Savvy Consumer’s Guide to Hearing Loss.” Our first guide was written nearly 20 years ago, and has been updated several times, the most recent in 2008, yet so much in our society has changed. -

Ear Problems and Treatments (PDF)
Ear problems and treatments “I knew from a young age that I couldn’t hear as well as I was supposed to – sometimes my Reception class teacher would think I was misbehaving when I didn’t follow her instructions, but I simply couldn’t hear what she was saying. I had two operations to insert grommets (ventilation tubes) in my ears, when I was eight and 10, to drain a build-up of fluid from the middle part of my ears and help prevent ear infections. The second operation eventually worked and, for the first time in my life, I could hear sounds clearly – the difference was amazing.” Tori Jeffery, West Yorkshire This leaflet tells you about the common ear conditions that can cause hearing loss or balance problems (or both) – and the treatments available. If you’re at all worried about your hearing or balance, see your GP. Contents • How our ears work ...................................................4 • Different types of hearing loss ........................................6 • Outer ear conditions ................................................. 7 • Excess ear wax ................................................... 7 • Otitis externa..................................................... 8 • Exostosis.........................................................9 • Middle ear conditions ................................................9 • Otitis media ......................................................9 • Glue ear ........................................................ 10 • Chronic suppurative otitis media (CSOM)...........................11 •