Preferred Drug List
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Potentially Harmful Drugs in the Elderly: Beers List
−This Clinical Resource gives subscribers additional insight related to the Recommendations published in− March 2019 ~ Resource #350301 Potentially Harmful Drugs in the Elderly: Beers List In 1991, Dr. Mark Beers and colleagues published a methods paper describing the development of a consensus list of medicines considered to be inappropriate for long-term care facility residents.12 The “Beers list” is now in its sixth permutation.1 It is intended for use by clinicians in outpatient as well as inpatient settings (but not hospice or palliative care) to improve the care of patients 65 years of age and older.1 It includes medications that should generally be avoided in all elderly, used with caution, or used with caution or avoided in certain elderly.1 There is also a list of potentially harmful drug-drug interactions in seniors, as well as a list of medications that may need to be avoided or have their dosage reduced based on renal function.1 This information is not comprehensive; medications and interactions were chosen for inclusion based on potential harm in relation to benefit in the elderly, and availability of alternatives with a more favorable risk/benefit ratio.1 The criteria no longer address drugs to avoid in patients with seizures or insomnia because these concerns are not unique to the elderly.1 Another notable deletion is H2 blockers as a concern in dementia; evidence of cognitive impairment is weak, and long-term PPIs pose risks.1 Glimepiride has been added as a drug to avoid. Some drugs have been added with cautions (dextromethorphan/quinidine, trimethoprim/sulfamethoxazole), and some have had cautions added (rivaroxaban, tramadol, SNRIs). -

Randomized Controlled Pilot Trial of Cabergoline, Hydergine and Levodopa/Carbidopa: Los Angeles Cocaine Rapid Efficacy Screening
Blackwell Science, LtdOxford, UKADDAddiction1359-6357© 2005 Society for the Study of Addiction 100•••• Original Article Cabergoline, hydergine, levodopa/carbidopa Steven Shoptaw et al. RESEARCH REPORT Randomized controlled pilot trial of cabergoline, hydergine and levodopa/carbidopa: Los Angeles Cocaine Rapid Efficacy Screening Trial (CREST) Steven Shoptaw1, Donnie W. Watson2, Chris Reiber1, Richard A. Rawson1, Margaret A. Montgomery3, Maria D. Majewska3 & Walter Ling1 UCLA Integrated Substance Abuse Programs, Los Angeles, CA1 , Friends Research Institute, Inc., Los Angeles, CA2 and National Institute on Drug Abuse, Division of Treatment Research and Development, Bethesda, MD, USA3 Correspondence to: ABSTRACT Steven Shoptaw PhD UCLA/Integrated Substance Abuse Programs Aim This study tested three dopaminergic medications against a common 11075 Santa Monica Blvd unmatched placebo condition: hydergine 1 mg three times daily (n = 15); Suite 200 levodopa/carbidopa 25/100 mg three times daily (n = 15); cabergoline 0.5 mg Los Angeles per week (n = 15); and placebo three times daily (n = 15) as potential pharma- CA 90025 USA cotherapies for cocaine dependence. E-mail: [email protected] Design The four-parallel group, Cocaine Rapid Efficacy Screening Trial (CREST) design featured a 2-week baseline period followed by randomization to an 8-week medication condition that included 1 hour per week of cognitive RESEARCH REPORT behavioral drug counseling. A safety evaluation was conducted 4 weeks after termination. Measures Outcomes included cocaine metabolites measured in urine, reten- tion and self-reports for drug use, cocaine craving, clinical improvement, mood and HIV risk behaviors. Results Participants assigned to receive cabergoline provided more urine sam- ples negative for cocaine metabolites (42.4%) than those assigned to receive pla- cebo (25.0%), a statistically significant difference after controlling for baseline differences in self-reported cocaine use (F = 2.95, df = 3; P = 0.05). -

Albert Hofmann's Pioneering Work on Ergot Alkaloids and Its Impact On
BIRTHDAY 83 CHIMIA 2006, 60, No. 1/2 Chimia 60 (2006) 83–87 © Schweizerische Chemische Gesellschaft ISSN 0009–4293 Albert Hofmann’s Pioneering Work on Ergot Alkaloids and Its Impact on the Search of Novel Drugs at Sandoz, a Predecessor Company of Novartis Dedicated to Dr. Albert Hofmann on the occasion of his 100th birthday Rudolf K.A. Giger* and Günter Engela Abstract: The scientific research on ergot alkaloids is fundamentally related to the work of Dr. Albert Hofmann, who was able to produce, from 1935 onwards, a number of novel and valuable drugs, some of which are still in use today. The complex chemical structures of ergot peptide alkaloids and their pluripotent pharmacological activity were a great challenge for Dr. Hofmann and his associates who sought to unravel the secrets of the ergot peptide alkaloids; a source of inspiration for the design of novel, selective and valuable medicines. Keywords: Aminocyclole · Bromocriptine Parlodel® · Dihydroergotamine Dihydergot® · Dihydro ergot peptide alkaloids · Ergobasin/ergometrin · Ergocornine · Ergocristine · α- and β-Ergocryptine · Ergolene · Ergoline · Ergoloid mesylate Hydergine® · Ergotamine Gynergen® · Ergotoxine · Lisuride · Lysergic acid diethylamide LSD · Methylergometrine Methergine® · Methysergide Deseril® · Paspalic acid · Pindolol Visken® · Psilocybin · Serotonin · Tegaserod Zelmac®/Zelnorm® · Tropisetron Navoban® Fig. 1. Albert Hofmann in 1943, 1979 and 2001 (Photos in 2001 taken by J. Zadrobilek and P. Schmetz) Dr. Albert Hofmann (Fig. 1), born on From the ‘Ergot Poison’ to *Correspondence: Dr. R.K.A. Giger January 11, 1906, started his extremely Ergotamine Novartis Pharma AG NIBR Global Discovery Chemistry successful career in 1929 at Sandoz Phar- Lead Synthesis & Chemogenetics ma in the chemical department directed The scientific research on ergot alka- WSJ-507.5.51 by Prof. -

2021 Formulary List of Covered Prescription Drugs
2021 Formulary List of covered prescription drugs This drug list applies to all Individual HMO products and the following Small Group HMO products: Sharp Platinum 90 Performance HMO, Sharp Platinum 90 Performance HMO AI-AN, Sharp Platinum 90 Premier HMO, Sharp Platinum 90 Premier HMO AI-AN, Sharp Gold 80 Performance HMO, Sharp Gold 80 Performance HMO AI-AN, Sharp Gold 80 Premier HMO, Sharp Gold 80 Premier HMO AI-AN, Sharp Silver 70 Performance HMO, Sharp Silver 70 Performance HMO AI-AN, Sharp Silver 70 Premier HMO, Sharp Silver 70 Premier HMO AI-AN, Sharp Silver 73 Performance HMO, Sharp Silver 73 Premier HMO, Sharp Silver 87 Performance HMO, Sharp Silver 87 Premier HMO, Sharp Silver 94 Performance HMO, Sharp Silver 94 Premier HMO, Sharp Bronze 60 Performance HMO, Sharp Bronze 60 Performance HMO AI-AN, Sharp Bronze 60 Premier HDHP HMO, Sharp Bronze 60 Premier HDHP HMO AI-AN, Sharp Minimum Coverage Performance HMO, Sharp $0 Cost Share Performance HMO AI-AN, Sharp $0 Cost Share Premier HMO AI-AN, Sharp Silver 70 Off Exchange Performance HMO, Sharp Silver 70 Off Exchange Premier HMO, Sharp Performance Platinum 90 HMO 0/15 + Child Dental, Sharp Premier Platinum 90 HMO 0/20 + Child Dental, Sharp Performance Gold 80 HMO 350 /25 + Child Dental, Sharp Premier Gold 80 HMO 250/35 + Child Dental, Sharp Performance Silver 70 HMO 2250/50 + Child Dental, Sharp Premier Silver 70 HMO 2250/55 + Child Dental, Sharp Premier Silver 70 HDHP HMO 2500/20% + Child Dental, Sharp Performance Bronze 60 HMO 6300/65 + Child Dental, Sharp Premier Bronze 60 HDHP HMO -
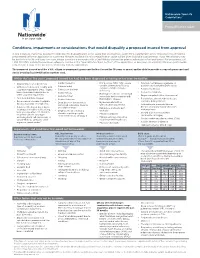
Conditions, Impairments Or Considerations That Would Disqualify a Proposed Insured from Approval
Nationwide YourLife CareMatters® Prequalification guide Conditions, impairments or considerations that would disqualify a proposed insured from approval In rare instances, there may be a history indicated for disqualification in this guide that you feel may qualify for a CareMatters® policy. These histories should be prescreened before an application is submitted. Histories not found in this prequalification guide will be given individual consideration. If you feel a history may be borderline for life and long-term care, please complete a prescreen with a CareMatters underwriter prior to submission of an application. For prescreens, call 1-855-381-5729. Include the prescreen reference number in the "Special Instructions Section" of the application, or prescreen via email at CMScreen@nationwide. com. Include a copy of the prescreen email reply with the application. The proposed insured must be a U.S. citizen or permanent green card holder (issued for 10 years or more) and be able to provide a copy of green card and Social Security/tax identification number card. Within the last five years, proposed insured has had, has been diagnosed as having or has been treated for: • Alcohol abuse or dependency • Cardiomyopathy • HIV positive, AIDS, ARC, severe • Paralysis, hemiplegia, paraplegia or • Cerebral palsy combined immunodeficiency, quadriplegia (excluding Bell's palsy) • Alzheimer's, dementia, senility, mild common variable immune • Cirrhosis of the liver • Parkinson's disease cognitive impairment (MCI), organic deficiency brain syndrome, -

EUROPEAN PHARMACOPOEIA 10.0 Index 1. General Notices
EUROPEAN PHARMACOPOEIA 10.0 Index 1. General notices......................................................................... 3 2.2.66. Detection and measurement of radioactivity........... 119 2.1. Apparatus ............................................................................. 15 2.2.7. Optical rotation................................................................ 26 2.1.1. Droppers ........................................................................... 15 2.2.8. Viscosity ............................................................................ 27 2.1.2. Comparative table of porosity of sintered-glass filters.. 15 2.2.9. Capillary viscometer method ......................................... 27 2.1.3. Ultraviolet ray lamps for analytical purposes............... 15 2.3. Identification...................................................................... 129 2.1.4. Sieves ................................................................................. 16 2.3.1. Identification reactions of ions and functional 2.1.5. Tubes for comparative tests ............................................ 17 groups ...................................................................................... 129 2.1.6. Gas detector tubes............................................................ 17 2.3.2. Identification of fatty oils by thin-layer 2.2. Physical and physico-chemical methods.......................... 21 chromatography...................................................................... 132 2.2.1. Clarity and degree of opalescence of -

Ergot Alkaloids: a Review on Therapeutic Applications
European Journal of Medicinal Plants 14(3): 1-17, 2016, Article no.EJMP.25975 ISSN: 2231-0894, NLM ID: 101583475 SCIENCEDOMAIN international www.sciencedomain.org Ergot Alkaloids: A Review on Therapeutic Applications Niti Sharma 1* , Vinay K. Sharma 1, Hemanth Kumar Manikyam 1 1,2 and Acharya Bal Krishna 1Patanjali Natural Coloroma Pvt. Ltd, Haridwar, Uttarakhand - 249404, India. 2University of Patanjali, Haridwar, Uttarakhand - 249402, India. Authors’ contributions This work was carried out in collaboration between all authors. Authors NS and VKS designed the study, wrote the first draft of the manuscript. Authors ABK and HKM supervised the study. All authors read and approved the final manuscript. Article Information DOI: 10.9734/EJMP/2016/25975 Editor(s): (1) Marcello Iriti, Professor of Plant Biology and Pathology, Department of Agricultural and Environmental Sciences, Milan State University, Italy. Reviewers: (1) Nyoman Kertia, Gadjah Mada University, Indonesia. (2) Robert Perna, Texas Institute of Rehabilitation Research, Houston, TX, USA. (3) Charu Gupta, AIHRS, Amity University, UP, India. Complete Peer review History: http://sciencedomain.org/review-history/14283 Received 28 th March 2016 Accepted 12 th April 2016 Review Article st Published 21 April 2016 ABSTRACT Ergot of Rye is a plant disease caused by the fungus Claviceps purpurea which infects the grains of cereals and grasses but it is being used for ages for its medicinal properties. All the naturally obtained ergot alkaloids contain tetracyclic ergoline ring system, which makes them structurally similar with other neurotransmitters such as noradrenaline, dopamine or serotonin. Due to this structure homology these alkaloids can be used for the treatment of neuro related conditions like migraine, Parkinson’s disease etc. -

Cover Next Page > Cover Next Page >
cover next page > Cover title: The Psychopharmacology of Herbal Medicine : Plant Drugs That Alter Mind, Brain, and Behavior author: Spinella, Marcello. publisher: MIT Press isbn10 | asin: 0262692651 print isbn13: 9780262692656 ebook isbn13: 9780585386645 language: English subject Psychotropic drugs, Herbs--Therapeutic use, Psychopharmacology, Medicinal plants--Psychological aspects. publication date: 2001 lcc: RC483.S65 2001eb ddc: 615/.788 subject: Psychotropic drugs, Herbs--Therapeutic use, Psychopharmacology, Medicinal plants--Psychological aspects. cover next page > < previous page page_i next page > Page i The Psychopharmacology of Herbal Medicine < previous page page_i next page > cover next page > Cover title: The Psychopharmacology of Herbal Medicine : Plant Drugs That Alter Mind, Brain, and Behavior author: Spinella, Marcello. publisher: MIT Press isbn10 | asin: 0262692651 print isbn13: 9780262692656 ebook isbn13: 9780585386645 language: English subject Psychotropic drugs, Herbs--Therapeutic use, Psychopharmacology, Medicinal plants--Psychological aspects. publication date: 2001 lcc: RC483.S65 2001eb ddc: 615/.788 subject: Psychotropic drugs, Herbs--Therapeutic use, Psychopharmacology, Medicinal plants--Psychological aspects. cover next page > < previous page page_ii next page > Page ii This page intentionally left blank. < previous page page_ii next page > < previous page page_iii next page > Page iii The Psychopharmacology of Herbal Medicine Plant Drugs That Alter Mind, Brain, and Behavior Marcello Spinella < previous page page_iii next page > < previous page page_iv next page > Page iv © 2001 Massachusetts Institute of Technology All rights reserved. No part of this book may be reproduced in any form by any electronic or mechanical means (including photocopying, recording, or information storage and retrieval) without permission in writing from the publisher. This book was set in Adobe Sabon in QuarkXPress by Asco Typesetters, Hong Kong and was printed and bound in the United States of America. -

August 2021 California Signaturevalue 4 Tier HMO Formulary
Pharmacy | Formulary | California 2021 California SignatureValue 4-Tier HMO Formulary Please note: This Formulary is accurate as of August 1, 2021 and is subject to change after this date. All previous versions of this Formulary are no longer in effect. Your estimated coverage and copay/coinsurance may vary based on the benefit plan you choose and the effective date of the plan. This Formulary can also be accessed online at myuhc.com > Pharmacy Information > Prescription Drug Lists > California plans > SignatureValue HMO plans. Plan-specific coverage documents may be accessed online at uhc.com/statedruglists > Small Group Plans > California. If you are a UnitedHealthcare member, please register or log on to myuhc.com, or call the toll-free number on your health plan ID card to find pharmacy information specific to your benefit plan. This Formulary is applicable to the following health insurance products offered by UnitedHealthcare: • SignatureValue • SignatureValue Advantage • SignatureValue Alliance • SignatureValue Flex • SignatureValue Focus • SignatureValue Harmony • SignatureValue Performance Updated 6/17/2021 6/21 © 2021 United HealthCare Services, Inc. All Rights Reserved. WF3890815-N Contents At UnitedHealthcare, we want to help you better understand your medication options. ..................................................... 3 How do I use my Formulary? ............................................. 4 What are tiers? ........................................................ 5 When does the Formulary change? ........................................ 5 Utilization Management Programs ......................................... 6 Your Right to Request Access to a Non-formulary Drug ....................... 6 Requesting a Prior Authorization or Step Therapy Exception ................... 7 How do I locate and fill a prescription through a retail network pharmacy? . 7 How do I locate and fill a prescription through the mail order pharmacy? . 7 How do I locate and fill a prescription at a specialty pharmacy? ............... -

First-Generation Antihistamines (Medications Commonly Used To
Highrisk medication reference sheet The Pharmacy Quality Alliance has determined the following medications have the highest risk of side effects among those 65 years of age or older. “Highrisk” means a medicine can cause serious health problems or accidents. Highrisk medications can be: • A medicine that raises your risk of drowsiness, confusion, depression, organ damage, serious harm from a fall, or other dangerous side effects. • A medicine for one health problem that worsens another health problem. • Two or more medications that are dangerous when taken together. The more medicines you take, the greater the risk of negative interactions. Please review the list of highrisk medications below. If you are taking one or more of the medications listed, please speak with your doctor to determine if there are safer choices with fewer possible side effects. Firstgeneration antihistamines (medications commonly used to treat allergies) • Brompheniramine • Cyproheptadine • Hydroxyzine • Carbinoxamine • Dexchlorpheniramine • Promethazine • Chlorpheniramine • Diphenhydramine (oral) • Triprolidine • Clemastine • Doxylamine AntiParkinson agents (to treat Parkinson’s disease) • Benztropine (oral) • Trihexyphenidyl Antithrombotics (medications used to prevent blood from clotting inappropriately) • Ticlopidine • Dipyridamole Antiinfective (medication used to treat infections) • Nitrofurantoin (only when taken for 90 days or more) Alpha blockers (medications that help blood vessels remain open) • Guanfacine • Reserpine (only if you take more than -
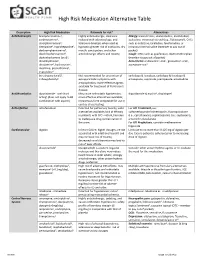
High Risk Medication Alternative Table
High Risk Medication Alternative Table Description High Risk Medication Rationale for risk* Alternatives Anticholinergics brompheniramine3, Highly anticholinergic, clearance Allergy: levocetirizine, desloratadine, montelukast, carbinoxamine3, reduced with advanced age, and azelastine, intranasal steroid (e.g., fluticasone4), OTCs chlorpheniramine3, tolerance develops when used as such as cetirizine, loratadine, fexofenadine, or clemastine3, cyproheptadine1, hypnotic; greater risk of confusion, dry intranasal normal saline (member to pay out of dexbrompheniramine3, mouth, constipation, and other pocket) dexchlorpheniramine3, anticholinergic effects and toxicity. Cough: OTCs such as guaifenesin, dextromethorphan diphenhydramine (oral)3, (member to pay out of pocket) dimenhydrinate3, Anti-emetic: ondansetron-oral1, granisetron-oral1, doxylamine3, hydroxyzine1, aprepitant-oral1 meclizine, promethazine1, triprolidine3 benztropine (oral)1, Not recommended for prevention of carbidopa & levodopa, carbidopa & levodopa & trihexyphenidyl1 extrapyramidal symptoms with entacapone, ropinirole, pramipexole, amantadine antipsychotics; more-effective agents available for treatment of Parkinson's disease. Antithrombotics dipyridamole - oral short May cause orthostatic hypotension; dipyridamole & aspirin4, clopidogrel acting1 (does not apply to ER more effective alternatives available; combination with aspirin) intravenous form acceptable for use in cardiac stress testing. Anti-infective nitrofurantoin Potential for pulmonary toxicity; safer For UTI Treatment, -

Modified Monoterpene Indole Alkaloid Production in the Yeast Saccharomyces Cerevisiae Copyright © 2017 by Amy M. Ehrenworth
MODIFIED MONOTERPENE INDOLE ALKALOID PRODUCTION IN THE YEAST SACCHAROMYCES CEREVISIAE A Dissertation Presented to The Academic Faculty by Amy M. Ehrenworth In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the School of Chemistry and Biochemistry Georgia Institute of Technology December, 2017 COPYRIGHT © 2017 BY AMY M. EHRENWORTH MODIFIED MONOTERPENE INDOLE ALKALOID PRODUCTION IN THE YEAST SACCHAROMYCES CEREVISIAE Approved by: Dr. Pamela Peralta-Yahya, Advisor Dr. Francesca Storici School of Chemistry and Biochemistry School of Biological Studies Georgia Institute of Technology Georgia Institute of Technology Dr. M.G. Finn Dr. Loren Williams School of Chemistry and Biochemistry School of Chemistry and Biochemistry Georgia Institute of Technology Georgia Institute of Technology Dr. Wendy L. Kelly School of Chemistry and Biochemistry Georgia Institute of Technology Date Approved: August 14, 2017 ACKNOWLEDGEMENTS I would like to thank all those who’ve guided me on my scientific and personal journey. I was blessed by the circumstances that have led me to this point, and I hope to make those who have influenced and inspired me proud. I want to express gratitude to my advisor Pamela Peralta-Yahya for all she has done for me in my time at Georgia Tech- from introducing me to the incredible field of synthetic biology and sharing her knowledge to helping make me a better scientist. I admire her dedication and ambition, and I am honored to have helped establish the Peralta-Yayha lab. I also appreciate the guidance I’ve received from all of my committee members throughout the years, be it via scientific discussions, mentorship, or personal inspiration.