ACR Appropriateness Criteria® Multiple Gestations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Monoamniotic Twins: What Should Be the Optimal Antenatal Management?
Monoamniotic Twins: What Should Be the Optimal Antenatal Management? Ashis K. Sau1, Kate Langford1, Catherine Elliott1, Lin L. Su2, and Darryl J. Maxwell1 1Fetal Medicine Unit, St.Thomas’ Hospital, London, UK 2National University Hospital, Singapore onoamniotic twinning is a rare event with an incidence of London with a high proportion of socially deprived and M1% of all monozygotic twins and associated with a high black, Asian or mixed race peoples. Management protocols fetal morbidity and mortality. Confident early diagnosis is possi- ble, but optimal management is not yet established. This article employed were those of early detection of chorionicity and presents the experience of a single centre in managing all amnionicity, 2-weekly serial ultrasound surveillance and monoamniotic twins diagnosed during 1994–2000. Seven pairs early delivery by elective cesarean section at around 32 of monoamniotic twins were identified for analysis. All were weeks gestation. From 1997, a formal twin ultrasound sur- managed in accord with a unit protocol that involved early diag- veillance clinic was established and first trimester nuchal nosis, serial ultrasound examination and elective early delivery. In four cases, the detection of monoamnionicity was made translucency screening was performed. The same fetal med- during a first trimester nuchal scan. Discordance for structural icine consultant had clinical input into the management of abnormality was found in three cases where the co-twin was all cases. A summary of the cases can be seen in Table 1. normal. Cord entanglement was detected antenatally in four cases. Two pairs of twins died before 20 weeks. One of these Case 1 had early onset twin–twin transfusion syndrome. -

The Natural History of Monoamniotic Twin Pregnancies: a Case Series and Systematic Review of the Literature
DOI: 10.1002/pd.4538 ORIGINAL ARTICLE The natural history of monoamniotic twin pregnancies: a case series and systematic review of the literature Federico Prefumo1*, Anna Fichera1, Giorgio Pagani1, Daria Marella1, Adriana Valcamonico1 and Tiziana Frusca1,2 1Maternal-Fetal Medicine Unit, Department of Obstetrics and Gynaecology, University of Brescia, Brescia, Italy 2Department of Obstetrics and Gynaecology, University of Parma, Parma, Italy *Correspondence to: Federico Prefumo. E-mail: [email protected] ABSTRACT Objective To describe the natural history of monoamniotic twin pregnancies in contemporary practice. Method Cohort study of monochorionic monoamniotic twin pregnancies with two live fetuses diagnosed at less than 16 weeks and prospectively followed up between 2004 and 2013. A systematic review of the literature using Medline, Embase and Scopus to determine the perinatal mortality rate after 24 weeks of gestation in monoamniotic twins was also performed. Results Twenty pregnancies were analyzed. Four were terminated (in three cases as a result of fetal abnormalities). Another six miscarried spontaneously. Among ten pregnancies reaching viability, there was double intrauterine death in one, and both fetuses were alive at delivery in the other nine. There were no neonatal deaths. Overall survival for fetuses alive at the initial scan was 18/40 (45%; 95% CI 29 to 62%). At meta-analysis of 13 studies (including the current series), the perinatal mortality rate after 24 weeks was 4.5% (95% CI 3.3 to 5.8%). Conclusions Despite early diagnosis and intensive monitoring, of those fetuses alive before 16 weeks less than half survive until the neonatal period. Most losses are attributable to fetal abnormalities and spontaneous miscarriage and are therefore unlikely to be reduced by further improvements in fetal assessment and monitoring. -

Fetal Distress Condition Twin-To-Twin Transfusion Syndrome (TTTS)
Fetal Distress Condition Twin-to-Twin Transfusion Syndrome (TTTS) Twice as many babies Description die from TTTS and TTTS or Twin-to-Twin Transfusion Syndrome is a disease of the placenta. It affects other fetal syndromes pregnancies with monochorionic (shared placenta) multiples when blood passes than from SIDS disproportionately from one baby to the other through connecting blood vessels within (Sudden Infant Death their shared placenta. One baby, the recipient twin, gets too much blood overloading his or Syndrome) each year; her cardiovascular system, and may die from heart failure. The other baby, the donor twin yet more people are or stuck twin, does not get enough blood and may die from severe anemia. Left untreated, mortality rates near 100%. aware of SIDS. The cause of TTTS is attributed to unbalanced flow of blood through vascular channels that connect the circulatory systems of each twin via the common placenta. The shunting of blood through the vascular communications leads to a net flow of blood from one twin (the donor) to the other twin (the recipient). The donor twin develops oligohydramnios (low amniotic fluid) and poor fetal growth, while the recipient twin develops polyhydramnios (excess amniotic fluid), heart failure, and hydrops. If left untreated, the pregnancy may be lost due to lack of blood getting to the smaller twin, fluid overload and heart failure in the larger twin, and/or preterm (early) labor leading to miscarriage of the entire pregnancy. Some general treatment approaches consist of using laser energy to seal off the blood vessels that shunt blood between the fetuses. -
Multiple Gestations
A Few Too Many Sonography of Multiple Gestations Ivana M Vettraino, MD, MBA Maternal Fetal Medicine Director, Perinatal Center, Genetics and Outreach Mercy – St Louis Disclosures Speakers bureau March of Dimes Hologic, Inc Trainer Nexplanon I will not be discussing any of these organizations or products in this presentation 2 Objectives Define and describe the categories of multiple gestations Describe the keys to ultrasound assessment of multiple gestation Describe sonographic complications of twin gestation and other higher order multiples Introduce management of twin gestation in the prenatal period Introduction Account for 3 % of all pregnancies Incidence human pregnancies Approximately 1 in 90 births Rate varies from country to country Highest numbers - Nigeria (45 twins per 1000 live births) Lowest in Japan (4 twins per 1000 live births) United States (2016) – 33.4 twins per 1000 live births Vary by ethnic group Hispanic women 19.5 per 1000 births Non-Hispanic black women 30.0 per 1000 births Non-Hispanic white women 28.8 per 1000 births Multiple Gestation Overall rise in multiple gestations Older age at childbearing 1/3 Increasing use of infertility therapies 2/3 Triplet Data Twinning Monozygotic twins (“identical”) Develop from a single fertilized ovum Have same genetic material Rate is constant worldwide 3.5 to 4 per 1000 births 30 percent of twins Dizygotic twins (“fraternal”) Develop from more than one fertilized ovum Genetically similar as any full siblings Rate varies by ethnic background, -

Monoamniotic Twins - Management
LOCAL OPERATING PROCEDURE – CLINICAL Approved Safety & Quality Committee May 2021 Review May 2026 MONOAMNIOTIC TWINS - MANAGEMENT This LOP is developed to guide clinical practice at the Royal Hospital for Women. Individual patient circumstances may mean that practice diverges from this LOP. 1. AIM • Diagnosis of monoamniotic twin pregnancy • Regular antenatal review, fetal welfare scanning and preterm birth plan • Preparation for parenting twins and preterm birth 2. PATIENT • Woman suspected of having a monoamniotic (MA) twin pregnancy 3. STAFF • Medical and midwifery staff • Sonographers • Neonatologists 4. EQUIPMENT • Ultrasound machine 5. CLINICAL PRACTICE • Diagnose the MA twin pregnancy by identifying two fetal poles with no separating membrane. The presence of one or two yolk sacs is no longer considered necessary for the diagnosis 1 • Arrange referral to Maternal fetal medicine (MFM) clinic whenever MA twins diagnosed • Discuss the need for increased antenatal surveillance with the woman, explain the increased incidence of unexpected stillbirth due to cord entanglement, fetal anomalies and preterm birth • Discuss screening for aneuploidy and fetal anomalies with first trimester screening or non- invasive prenatal screening plus structural ultrasound scan • Recommend fortnightly ultrasound from 16 weeks gestation and one to two weekly antenatal ultrasound from 24 weeks gestation to monitor: o Position of fetuses o Cord entanglement o Amniotic fluid volume o Umbilical artery Doppler blood flow o Middle cerebral artery Doppler waveforms (from 20 weeks gestation) o Growth (on a fortnightly basis) o Fetal bladder volume • Discuss options of frequency of ultrasound and cardiotocograph (CTG) monitoring, including inpatient and outpatient monitoring • Recommend outpatient management from 26 weeks if Monochorionic Monoamniotic (MCMA) twins are uncomplicated • Consider CTG monitoring from 26 weeks gestation as part of outpatient management (see educational notes), particularly if there is significant estimated fetal weight discordance …./2 2. -
Multiple Pregnancies
Learning Objectives Using Ultrasound to Manage Twins After this presentation, the learner will be able to discuss: • Diagnosis and dating in twin pregnancies • Sonographic characteristics that distinguish dichorionic from monochorionic twins • Prenatal ultrasound screening in twins • Complications unique to monochorionic twins Lynn L. Simpson, MD Chief, Division of Maternal Fetal Medicine • Ultrasound surveillance recommendations for twins Columbia University Medical Center, New York Diagnosis and Dating of Twins Importance of Pregnancy Dating • Diagnosis best in the first trimester and dating optimal using crown-rump length • Timing for screening − 20% of first trimester twin pregnancies result in singleton live births and diagnostic testing − “Vanishing twin” is associated with favorable prognosis of surviving twin if dichorionic • Accurate interpretation of twin growth • If discrepancy in dates between the twins, date using the larger twin • Scheduling of twin deliveries − Avoids missing diagnosis of IUGR Types of Twins Determination of Chorionicity • All dizygotic twins are dichorionic • All monochorionic twins are monozygotic • Not all monozygotic twins are monochorionic • Optimal in first trimester − close to 100% accuracy • Incorrect assignment in up to 10% of cases when chorionicity determined in second trimester 2-3 days 3-8 days 8-13 days (28%) (70%) (1%) Lee et al, 2006 Blumenfeld et al, 2014 1 Determination of Chorionicity Intertwin Membrane • Gestational sacs • Amniotic sacs • Placenta number • Intertwin membrane • Gender -

Management of Twin Pregnancy
DOI: 10.1002/ijgo.12742 FIGO COMMITTEE REPORT Good clinical practice advice: Management of twin pregnancy FIGO Working Group on Good Clinical Practice in Maternal–Fetal Medicine*,a *Correspondence: Gian Carlo Di Renzo, Department of Obstetrics and Gynecology, University of Perugia, Santa Maria della Misericordia University Hospital, Perugia, Italy. Email: [email protected] Endorsed in March 2017 and April 2018 by the FIGO Executive Board. This advice should not be considered as standards of care or legal standards in clinical practice. aWorking Group members and expert contributors are listed at the end of the paper. PREMISE pregnancies presenting later than 14 weeks’ gestation should be dated according to the head circumference of the larger twin.6 Twin pregnancy is associated with a high risk of perinatal, as well as maternal, mortality and morbidity.1–3 Intensive antenatal fetal surveil- Determining chorionicity and amnionicity of lance is associated with a lower risk of stillbirth.4 The excess perinatal twin pregnancies mortality and morbidity is higher in monochorionic than dichorionic twin pregnancy due to the placental anastomoses invariably present The chorionicity and amnionicity should be determined in the first in the monochorionic placenta.5 This guideline will cover the care of trimester. The chorionicity is determined by examining the mem- both uncomplicated and complicated twin pregnancies. brane thickness at the site of their insertion into the placenta; a T sign indicates monochorionicity, while a lambda (λ) sign -

Multiple Pregnancy: Having More Than One Baby About This Information a Multiple Pregnancy Means You Are Having More Than One Baby at the Same Time
Information for you Published in September 2021 Multiple pregnancy: having more than one baby About this information A multiple pregnancy means you are having more than one baby at the same time. This is most commonly twins but may include triplets or, rarely, more. This information is for you if you are having a multiple pregnancy. It may also be helpful for your partner, family or friends. It focuses on twin and triplet pregnancies. If you are having more babies you can still use this information but you will have an individualised plan of care for your pregnancy depending on your circumstances. The information here aims to help you better understand your pregnancy and your options for treatment and care. Your healthcare team is there to support you in making decisions that are right for you. They can help by discussing your situation with you and answering your questions. 1 A glossary of medical terms is available on the RCOG website at: www.rcog.org.uk/en/patients/medical-terms. Key Points • Multiple pregnancy happens in about one in 60 pregnancies. • Most women with a multiple pregnancy will have a healthy pregnancy and will give birth to healthy babies, however complications are more common. • You will be offered extra antenatal checks and ultrasound scans to make sure that you are well and to monitor your babies closely. • If you have a multiple pregnancy you are more likely to give birth to your babies prematurely. • You will be advised to give birth in hospital. What is a multiple pregnancy? A multiple pregnancy is the term used when you are expecting two or more babies at the same time (twins, triplets or more). -

Monochorionic Monoamniotic Twins: Neonatal Outcome
Journal of Perinatology (2006) 26, 170–175 r 2006 Nature Publishing Group All rights reserved. 0743-8346/06 $30 www.nature.com/jp ORIGINAL ARTICLE Monochorionic monoamniotic twins: neonatal outcome L Cordero1, A Franco2 and SD Joy2 1Department of Pediatrics and Obstetrics, Division of Neonatal-Perinatal Medicine, College of Medicine and Public Health, The Ohio State University Medical Center, The Ohio State University, Columbus, OH, USA and 2Department of Obstetrics and Gynecology, College of Medicine and Public Health, The Ohio State University, Columbus, OH, USA Introduction Background: Monochorionic monoamniotic twins (MoMo) occur in one Every year 4 million infants are born in the United States and of 10 000 pregnancies. Cord entanglement, malformations, twin-to-twin approximately 130 000 of them are twins.1 The prevalence of twin transfusion syndrome (TTS) and prematurity are responsible for their high deliveries has increased 48% between 1990 and 1998.1 Of great perinatal morbidity and mortality. concern is that although twins represented only 2.5% of the entire Objective: To report our experience with 36 sets of MoMo twins (1990 to group, they accounted for 12.6% of the total perinatal mortality.2 2005) and to provide updated information for counseling. Only one-third of all twins are monozygotic, but 75% of the Methods: Chorionicity was determined by placental examination, monozygotic twins are monochorionic. Among monochorionic gestational age and TTS clinically and by sonography. Intrauterine twins, about 2% are monochorionic monoamniotic (MoMo), 18% growth restriction (IUGR) was diagnosed with a twin-specific nomogram. monochorionic diamniotic (MoDi) with twin-to-twin transfusion syndrome (TTS) and 80% MoDi without TTS.3 Monochorionic Results: Cord entanglement was observed in 15 pregnancies, but only monoamniotic twins have the highest incidence of perinatal losses, one twin with entanglement and a true knot, experienced related congenital malformations, perinatal mortality and perinatal morbidity. -

Monoamniotic Twins
Information for Women Bogulkunta, Hyderabad - 500 001 Ph : 040 - 40222300 Fax : 040 - 24753482 Email : [email protected] Web : www.fernandezhospital.com What is a Multiple Pregnancy? A multiple pregnancy is a pregnancy with two or more babies. The different terms used depend on the number of babies: Two : Twin Three : Triplets Four : Quadruplets Five : Quintuplets Six : Sextuplets Seven : Septuplets Multiples account for only a small percentage (3%) of all births, but the multiple pregnancy rate is rising. Why are Multiple Pregnancies Increasing? The reason why a woman has a multiple pregnancy may be due to many factors. A few important ones are : Hereditary : A family history increases the chance of having a multiple pregnancy. Maternal Age : About one-third of the increase in multiple pregnancies is due to the fact that more women over age 30 are having babies. Women in this age group are more likely than younger women to conceive multiples. Assisted Reproduction : Medicines that stimulate ovulation help produce many eggs, which if fertilized can result in a multiple pregnancy. In-vitro fertilization, during which eggs are removed from the mother, fertilized in the lab and then transferred to the uterus, result in the transfer of many fertilized eggs into the uterus, thus resulting in a multiple pregnancy. 2 Types of Twin Pregnancy There are two kinds of twins. Non-identical (dizygotic) : Eighty percent of all twins are non-identical. That means they come from two eggs with two sperms fertilizing them. These twins are as similar as siblings can be, of the same or different sex. They have their own placenta (dichorionic) and pregnancy sacs (diamniotic) and are called Dichorionic Diamniotic (DCDA ) twins. -

Twins: New Guidelines Objective Question 1
Twins: New Guidelines What you need to know Lynn L. Simpson, MD Chief, Division of Maternal Fetal Medicine Columbia University Medical Center, New York Objective • To review the expected “standard of care” when managing twin pregnancies − to provide high-quality obstetric care − to avoid medical malpractice Question 1 Which of the following is most predictive of a twin pregnancy? a. Larger than expected uterine size b. Strong family history of twins c. Severe hyperemesis d. Elevated serum βHCG e. Conceived via ovulation stimulation 1 Clinical Suspicion of Twins • Larger than expected uterine size • Strong family history of fraternal twins • Severe hyperemesis gravidarum • Elevated serum βHCG • Assisted reproductive technologies Prior to routine use of ultrasound, 10-15% of twins diagnosed in labor Diagnosis and Dating of Twins • Diagnosis best in the first trimester and dating optimal using crown-rump length − 20% of first trimester twin pregnancies result in singleton live births − Vanishing twin associated with favorable prognosis of surviving twin, similar to singleton pregnancy • Multiple biometric measurements should be used beyond the first trimester • If discrepancy in dates between the twins, date using the larger twin Landy et al, 1986 Importance of Pregnancy Dating in Twins • Timing for screening and diagnostic testing • Accurate interpretation of twin growth • Scheduling of twin deliveries 2 Question 2 Which of the following is most characteristic of dichorionic twins? a. Single placenta b. Same gender c. Two amnions d. T-sign -
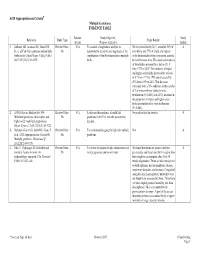
ACR Appropriateness Criteria® Multiple Gestations EVIDENCE TABLE
ACR Appropriateness Criteria® Multiple Gestations EVIDENCE TABLE Patients/ Study Objective Study Reference Study Type Study Results Events (Purpose of Study) Quality 1. Kulkarni AD, Jamieson DJ, Jones HW, Review/Other- N/A To conduct a longitudinal analysis to We estimated that by 2011, a total of 36% of 4 Jr., et al. Fertility treatments and multiple Dx determine the trends in and magnitude of the twin births and 77% of triplet and higher- births in the United States. N Engl J Med. contribution of fertility treatments to multiple order births resulted from conception assisted 2013;369(23):2218-2225. births. by fertility treatments. The observed incidence of twin births increased by a factor of 1.9 from 1971 to 2009. The incidence of triplet and higher-order births increased by a factor of 6.7 from 1971 to 1998 and decreased by 29% from 1998 to 2011. This decrease coincided with a 70% reduction in the transfer of 3 or more embryos during in vitro fertilization (P<0.001) and a 33% decrease in the proportion of triplet and higher-order births attributable to in vitro fertilization (P<0.001). 2. ACOG Practice Bulletin No. 144: Review/Other- N/A To discuss the incidence of multifetal No results stated in abstract. 4 Multifetal gestations: twin, triplet, and Dx gestations in the U.S. over the past several higher-order multifetal pregnancies. decades. Obstet Gynecol. 2014;123(5):1118-1132. 3. DeJesus Allison SO, Javitt MC, Glanc P, Review/Other- N/A To recommend imaging for high risk multiple N/A 4 et al.