Profile Prof. Gopal C. Kundu Has Obtained His Ph.D. in Chemistry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cover Legend: Gopal Kundu; a Member of the Editorial Academy of the International Journal of Oncology
INTERNATIONAL JOURNAL OF ONCOLOGY 58: MAY, 2021 Cover legend: Gopal Kundu; a member of The Editorial Academy of The International Journal of Oncology the area of research specific to disease known as ‘Coronary Artery Restenosis after angioplasty’ and published a study on this topic (Potential roles of osteopontin and avb3 integrin in the development of coronary artery restenosis after angioplasty. Proc Natl Acad Sci USA 94: 9308‑9313,1997). In addition, he filed a US patent along with his team on ‘Methods and compositions for treatment of restenosis. US Patent No. US 6,458,590 B1' in 2002 and received a Fellows Award for Research Excellence (FARE) for best research from NIH, USA in 1997. Professor Kundu subsequently returned to India in 1998 and Professor Gopal C. Kundu, was born into a very poor family acquired the position of Scientist‑D (equivalent to Assistant in a remote village of West Bengal, India. He completed his Professor) at the National Centre for Cell Science (NCCS), Pune, undergraduate (1977‑1980) and postgraduate (1980‑1983) India under the Ministry of Science and Technology, Government studies in Chemistry at the University of Calcutta, Kolkata, India of India. He was then promoted to Scientist‑E, Scientist‑F and and then obtained his Ph.D. degree in Chemistry from Bose then Scientist‑G (equivalent to Professor). He spent his valuable Institute (Sir J. C. Bose), Kolkata, India in 1989. time at NCCS, Pune, India from 1998 to 2019 and his area of Professor Kundu undertook his post‑doctoral research at research at NCCS was in the area of Tumor biology, cancer stem the Department of Heart and Hypertension (now known as the cells, tumor‑stromal interaction, angiogenesis, cancer Department of Molecular Cardiology), The Cleveland Clinic therapeutics, biomarker development and nanomedicine. -

1 Psi News Letter January-2019
PSI NEWS LETTER 1 JANUARY-2019 CONTENTS SECTION NAME PAGE NO’S 1. EDITOR’S MESSAGE............................................... ......................................4 2. REPORT OF PSI, 10TH ANNUAL MEETING.................................................5 - 11 3. CPG WORKSHOP, IIT BOMBAY...................................................................12 - 14 4. JPP: “SPRINGS” TO THE INTERNATIONAL STAGE ...............................15 5. MRM CONSORTIUM: A PSI INITIATIVE................................................16 6. RESEARCH UPDATES.................... .............................................................. 17-19 7. PSI - 2018 AWARD WINNERS.......... .............................................................20-26 8. UPCOMING WORKSHOPS & EVENTS........................................................27-31 PSI NEWS LETTER 2 JANUARY-2019 PSI EXECUTIVE COMMITTEE President Member Dr. Utpal S Tatu Dr. Rakesh K Mishra Vice President Dr. Abhijit Chakrabarti Dr. Subhra Chakraborty Dr. Shantanu Sengupta Dr. Arun Bandyopadhyay Dr. K Dharmalingam Gen. Secretary Dr. Kalpana Bhargava Dr. Mahesh J Kulkarni Dr. Niranjan Chakraborty Dr. Amol R Suryawanshi Treasurer Dr. M.V Jagannadham Dr. Suman S. Thakur Dr. Harsha Gowda Jt. Secretary Dr. Srikanth Rapole Dr. Sanjeeva Srivastava Dr. Suman Kundu Past President of PSI Dr. Ashok Kumar Mohanty Dr. Ravi Sirdeshmukh Dr. Sharmila Chattopadhyay (Founder President, EC member) Dr. Debasis Dash Dr. Surekha Zingde (Immediate Past President, ex officio member of EC) PSI NEWS LETTER 3 JANUARY-2019 -

Past Seminars Upto 2020
Past Seminars upto 2020 Sat December 5 2020 10:30 - 11:30 DBS Seminar: Dr. Rupasri Ain (CSIR-Indian Institute of Chemical Biology); Talk Title: "MicroRNAs in trophoblast development and placental disorder" Where: Asima Chatterjee Lecture Theatre Tue March 17 2020 12:00 - 13:00 DBS Seminar: Prof. Svetlana Lutsenko (Dept of Physiology, Johns Hopkins University, School of Medicine, Baltimore, US); Talk Title: Wilson disease: the mechanism and new strategies for treatment Where: Meghnad Saha Lecture Theatre Mon March 16 2020 15:00 - 16:00 DBS Seminar: Prof. Svetlana Lutsenko (Dept of Physiology, Johns Hopkins University, School of Medicine, Baltimore, US); Talk Title: Copper-transporters at the intersection of cell metabolism and differentiation Where: Meghnad Saha Lecture Theatre Wed March 11 2020 15:00 - 17:00 DBS student talk: Ms. Rubina Mondal; Talk Title: Beta diversity in freshwater fishes: drivers and mechanisms Where: Asima Chatterjee Lecture Theatre Fri March 6 2020 15:00 - 16:15 DBS Seminar: Prof. Samudrala Gourinath, School of Life Sciences, Jawaharlal Nehru University; Talk Title: Structural and functional studies of Myosin IB: Understanding the role in phagocytic cup formation inEntamoeba histolytica Where: S N Bose Lecture Theatre Thu February 27 2020 10:00 - 11:00 Pre-submission Open Seminar on 27th February, Spaker: Ms. Shritama Aich, IISER Kolkata; Talk Title:Towards efficient cellulolytic hydrolysis: Characterization and engineering of thermostable endoglucanases Wed February 12 2020 11:00 - 12:00 Pre-submission Open Seminar on 27th February, Spaker: Mr. Aditya Ghoshal, IISER Kolkata; Talk Title:Male-female interactions and mating strategies in wild zebrafish (Danio rerio) Where: 3rd Floor TRC DBS Meeting Room (N315C) Mon February 3 2020 12:00 - 13:00 DBS Seminar: Dr. -

GREF CENTRE PUNE LIST of CANDIDATES FOUND NOT ELEGIBLE Following Candidates Are Found Not Eligible for Issue of Call Letters
ADVT 01/2010 Page No. 1 LIST OF CANDIDATES FOUND NOT ELEGIBLE ADVT- 01/2010 TRADE -SAFAIWALA CAT-OBC TOTAL CANDIDATES - 5452 Following candidates are found Not Eligible for issue of Call Letters due to one or more of the following reasons: No reserved vacancy, Less than cut off mark, Less Qualification, Less or NO Experience, Residence certificate not attached, Photo not attested, Photo not affixed on Admit Card, Over or Under Age, Documents not attached, Incomplete Application, Caste Certificate not attached, Without application fee, Date of Birth proof not attached, Marks sheet not attached, Certificate not attested, Valid Caste Certificate not attached. Srl Control No Name Father's Name DOB No. GREF CENTRE PUNE 1 S/WALA/OBC/PH/31 NARASIMHA SWAMY NAGARAJU 4-Aug-88 6638 NAGIDI 2 S/WALA/OBC/31500 WALDE VINOD WALDE SHENAFADU 20-Nov-84 2 SHENAGADU PINDAKLIK 3 S/WALA/OBC/31500 SANGALE VIKAS SANGALE BHAUSAHEB 28-Mar-92 3 NBHAUSAHEB KESHAV 4 S/WALA/OBC/31500 BIJU V VAMADEVAN 16-Mar-86 8 5 S/WALA/OBC/31501 JAYAN C CHANDRAN 5-May-84 0 6 S/WALA/OBC/31501 SAKHARE SAKHARE RAMESH 8-Feb-90 6 DAHANANJAY PARASRAM RAMESH 7 S/WALA/OBC/31502 SENNY VIJAYAN K VIJAYAKUMAR 1-Apr-88 0 8 S/WALA/OBC/31502 SANDEOP V VIJAYAN 17-Jan-90 2 9 S/WALA/OBC/31502 TILEKAR GANESH TILEKAR 1-Aug-89 6 CHANDRAKANT CHANDRAKANT VASANT 10 S/WALA/OBC/31503 SINKAR UMESH SINKAR VITTHAL 15-Aug-86 1 VITTHAL ANANDA 11 S/WALA/OBC/31503 MAHAJAN PRAMOD MAHAJAN BADRINATH 1-Jan-90 2 BADRINATH SAMBHAJI 12 S/WALA/OBC/31504 TAMBOLI SHABBIR TAMBOLI SHEKHALAL 26-Mar-88 0 SHEKHALAL BABALAL 13 S/WALA/OBC/31504 PALVE SANDIP PALVE BABURAO 26-Jan-87 3 BABURAO SANHEBRAO ADVT 01/2010 Page No. -

Case Summary & Final Proceedings of SSV on the Kundu-JBC Case 28
Case Summary & Final Proceedings of SSV on the Kundu-JBC case 28 April, 2007 In August 2006, SSV received a signed complaint from Prof. Sohan P. Modak, a retired professor from the Dept of Zoology, University of Pune, alleging that Dr. Gopal Kundu and his coworkers (Hema Rangaswami and Anuradha Bulbule) from the National Centre for Cell Science (NCCS), Pune, India, published some papers in the prestigious Journal of Biological Chemistry, by —fraudulent reuse of the Western Blot data“. The locus standi of Prof. Modak is that he had a close association with the institute since its inception, as the Principal Co- Investigator/Founder of the NFATCC (subsequently renamed as NCCS), the first Research Director of NFATCC and a former member of the Governing Council of NCCS. His complaint also mentioned that even though an internal committee of the institute indicted the authors, the Director appointed another external committee, which exonerated the authors of all the charges and suspected complicity by the second committee chaired by Prof. G.Padmanabhan. This committee had several prominent scientists from different parts of India as its members, such as Dr. Kanury Rao, Dr. Dinakar Salunke, Dr. Umesh Varshney, Dr. Anil Tyagi, Dr. Shekhar Mande and Dr. Islam Khan. SSV‘s queries revealed that the first internal committee comprising of scientists from NCCS apparently held the authors guilty and sought withdrawal of the JBC papers (though no official record of it has been made available to SSV despite repeated requests). Kundu even confessed in writing about the problems in those papers and agreed to their withdrawal, but retracted his confession soon thereafter to claim innocence and accuse the committee of extracting a confession from him under duress. -

(IDIP-2021) 22Nd to 24Th April 2021 Organized by Department of Biotechnology, Savitribai Phule Pune University Programme Schedule
International Conference on Infectious Diseases and Immunopathology (IDIP-2021) 22nd to 24th April 2021 Organized by Department of Biotechnology, Savitribai Phule Pune University Programme Schedule Day-1: 22.04.2021 (Thursday) Sl.No. Time Speakers Title of Presentation 9:00 AM 9:40 AM Opening Ceremony 9:40 AM 10:00 AM Tea Break Chairperson: Prof. Surendra Ghaskadbi 1 10:00 AM 10:30 AM Prof. Chyung-Ru Wang CD1-restricted Lipid Antigen Northwestern University, USA Specific T Cell Responses in Mycobacterium tuberculosis Infection 2 10:30 AM 11:00 AM Dr. Prashant Kodgire Dual role of HomA and HomB, IIT-Indore outer membrane proteins of H. pylori, in suppression of B-cell antibody diversity and expression of T-cell inhibition markers 3 11:00 AM 11:30 AM Prof. Shailja Singh Pore Forming Proteins of Jawaharlal Nehru University Plasmodium Triggers the Necrosis of Endothelial Cells and contribute to Malaria Severity 11:30 AM 12:00 PM Tea Break Chairperson: Prof. S. Mohan Karuppayil 4 12:00 PM 12:30 PM Dr. Rupinder Kaur Functional genomic approaches The Centre for DNA to understand the pathobiology of Fingerprinting and Diagnostics Candida glabrata (CDFD) 5 12:30 PM 1:00 PM Dr Harinath Chakrapani Towards Identifying Indian Institute of Science Vulnerabilities in Bacteria through Education and Research, Pune Protein Profiling 6 1:00 PM 1:30 PM Dr. Benu Brata Das New Players of DNA Indian Association for The Topoisomerase I-induced DNA Cultivation of Science, Kolkata damage and repair. 1:30 PM 2:30 PM Lunch Break 7 2:30 PM 4:30 PM Poster Presentations by Attendees Day-2: 23.04.2021 (Friday) Chairperson: Dr. -

7Th Chornicle(3)
ISSUE VII • MAY 2019 #1 PVT. UNIVERSITY IN HARYANA AS PER INDIA TODAY ‘INDIA'S BEST UNIVERSITIES’ PADMA VIBHUSHAN DR RAGHUNATH ANANT “MY VISION IS TO MASHELKAR & ADITYA BIRLA GROUP CHAIRMAN MAKE INDIA KNOWLEDGE SUPER KUMAR MANGALAM BIRLA CONFERRED WITH POWER BY 2030" TH HONORARY DOCTORATE AT 5 CONVOCATION Dr. Ashok K. Chauhan Founder President Amity Education Group MESSAGES MESSAGE FROM CHANCELLOR It gives me immense pleasure and a sense of great pride to mention that Amity, being always on the front foot of research and innovation, has established the 'Capacity Building Center' as part of the Amity Center for Academic Innovation in Education. Padma Vibhushan Dr. R A Mashelkar and Aditya Birla Group Chairman A total of 1,456 graduands of various programs, including Engineering Shri Kumar Mangalam Birla were bestowed with Honorary Doctorate & Technology, Management, Applied Sciences, Journalism & The Capacity Building Center with hi-tech by Dr. Ashok K. Chauhan, Founder President, Amity Education Group, Mass Communication, Medical Sciences, Languages and Commerce, facilities for lecture deliver y and during the fifth convocation ceremony of the university on the received UG and PG degrees; besides 59 Gold Medals, 58 Silver computational facility aims to improve the university campus, Panchgaon, Manesar, on Saturday. Medals and 47 Bronze Medals were bestowed on the top rankers. quality of teaching in Higher Education 20 students were awarded Ph.Ds and meritorious students from Institutes by providing Capacity Building Accepting the honor, Shri Kumar Mangalam Birla congratulated different institutions were felicitated with 6 Dr. Ashok K. Chauhan solutions for the teaching fraternity. It will also serve as a platform for Amity University for adding a new dimension to the field of education. -

20Th Transcription Assembly Meeting 2018 25Th-27Th July 2018 Centre for DNA Fingerprinting and Diagnostics, Hyderabad
20th Transcription Assembly Meeting 2018 25th-27th July 2018 Centre for DNA Fingerprinting and Diagnostics, Hyderabad Conference Program DAY 1 - 25th July 8:45 - 9:15 Arrival and registration 9:15 - 9:25 Debashis Mitra (Director, CDFD) Inauguration and Welcome address 9:25 - 13:15 Moderator: Deepti Jain (RCB, Delhi) 9:25 - 10:50 Dipankar Chatterjee (IISc, Bangalore) " Comprehensive Analysis of two Iron Storage Proteins: A case study with Dps and Ferritin" 9:50 - 10:15 Gowrishankar J(CDFD, Hyderabad) “Antisense transcripts that generate toxic R-loops in E. coli are themselves Rendered undetectable because of the R-loops so formed” 10:15 - 10:40 Jayanta Mukhopadhyay (Bose Institute, Kolkata) " Direct evidence of robustness in a two-component system 10.40 - 11.05 Vikas Jain (IISER, Bhopal) “Dissection of the factors responsible for the stability and activity of T4 latepromoter transcription effector gp45” 11:05 - 11:25 Tea 11:25 - 11:50 Dipak Dutta (IMTech, Chandigarh) " Rho is a potential regulator of trace metal homeostasis in Escherichia coli" 11:50 – 12:15 Deepti Jain (RCB, Delhi) “Transcription Regulation of flagellar gene network in Pseudomonas Aeruginosa” 12:15 –12:40 Harinarayanan(CDFD, Hyderabad) “SpoT and GppA hydrolases prevent the activation of RelA by pppGppin Escherichia coli” 12:40 –13:05 Jitender Thakur (JNU, Delhi) “KIX domain containing Mediator subunit Med15 is important for transcriptional regulation of seed development in rice” 13:05- 14:00 Lunch 14:00 - 15:00 Poster session 15:00 - 15:20 Tea 15:20 - 18:30 Moderator: -
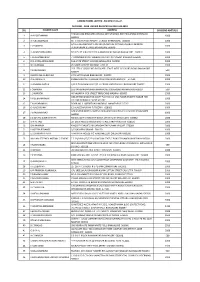
Srl Holder Name Address Dividend Amt (Rs.) 1 a A
CANFIN HOMES LIMITED - EQ NEW FV Rs.2/- DIVIDEND - 2018 UNPAID REGISTER AS ON 31-03-2019 SRL HOLDER NAME ADDRESS DIVIDEND AMT (RS.) TADKOD ONI BHUSAPPA CHOWK OPP SHARADA PRINTING PRESS DHARWAD 1 A A DODDAMANI 1000 580001 2 D ALBUQUERQUE 65 VIVEKANANDA NAGAR 1 CROSS BANGALORE. 560033 1000 S/O E.S.GURUBASAPPA NO 2942/E MAITRE OPPOSITE MARUTI MANDIR 3 E G RAMESH 1000 VIJAYANAGAR II STAGE BANGALORE 560040 4 H A KANTHARAJAPPA NO 375 IST N BLOCK 19TH G MAIN RAJAJI NAGAR BANGALORE 560010 1000 5 L CHIDHAMBARAM 7 MANAGIRI ROAD SUBBIAH COLONY K K NAGAR MADURAI 625020 1000 6 O D GOPALAKRISHNAN 16A G5TH STREET ULSOOR BANGALORE 560008 1000 7 W H KANNAN 45 NORTH STREET WATRAP 626132 1000 259 17TH C CROSS 3RD BLOCK 4TH STAGE WEST OF CHORD ROAD BANGALORE 8 Y R JAYASIMHA 500 560079 9 ZACKIRUNNISA BEGUM 47/1 MOTINAGAR BANGALORE 560002 2000 10 D B ANJANEYA KUMBALUR POST HARIHAR TOLUK CHITRADURGA DIST 577530 1000 11 H A MANJUNATHA C/O D THIAGARAJ NO.15/2 III CROSS MATADAHALLI BANGALORE 560032 1000 12 J CHANDRA 163 RANGARAJAPURAM MAIN ROAD KODAMBAKKAM MADRAS 600024 500 13 L D KAPOOR 40 NAGAPPA IYER STREET TRIPLICANE MADRAS 600005 2500 OM ASHTAGANDA HSG SOCTY FLAT NO.14 2ND FLOOR SHASTRI NAGAR RBI 14 Y R SUBRAMANIAN 1000 COLONY DOMBIVILI WEST 421202 15 E N JAYARAMULU DOOR NO. 1-88FORT KALYAN DRUG ANANTAPUR 515761 2000 16 G ALAGIRISAMY 12 ALAGESAPURAM TUTICORIN 628002 1000 C/O UNITED DENTAL SUPPLY CO SAJJANRAO CIRCLE V V PURAM BANGALORE 17 I V RAMANA RAO 1000 560004 18 J D SENANI SUBRAMANYA NO 435 44TH CROSS 8TH BLOCK JAYANAGAR BANGALORE 560082 2000 19 M A -

5Th ICTR-2019 Flyer.Ppt
Date : 07th – 09th November, 2019 Venue : National Centre for Cell Science, Pune, India 5th International Conference on Translational Research:“Recent Trends in Tentative / Confirmed Speakers th th Prof. G. K. Rath, India Pre-translational to Translational Research” will be held from 07 – 09 Prof Lucio Miele, USA Prof Sankar Ghosh, USA November, 2019 at National Centre for Cell Science, Pune, India. Many Prof. Srikumar Chellappan, USA distinguished researchers will present their exciting work as Plenary and Prof. Priyabrata Mukhopadhyay, USA Dr. Subhra Chakraborty, India Invited lectures and short oral presentation. Students and post-doctoral Dr. Amit Dinda, India fellows will present their findings as posters and the extraordinary works will Dr. Arvind Sahu, India Dr. Prabhansu Tripathi, India be recognized in the form of short oral presentations and poster awards. Prof. N. C. Talukdar, India Dr. Ramesh Sonti, India Jointly Organized by: Dr. Sharmila Bapat, India Dr. Sanjeev Galande, India National Centre for Cell Science (NCCS), Pune , India Dr. Soumitra Das India Dr. Sagar Sengupta, India Indian Society of Translational Research (ISTR), New Delhi , India Dr. Shantanu Choudhury, India Thematic Areas Dr. Tapas Kundu, India v v Dr. Jomon Joseph, India Stem Cell Biology Plant Biology Dr. Anjan Banerji, India v Drug Discovery v Regenerative Medicine Dr. Kumar Somasundaram, India v Diagnostics & Therapeutics v Pre-Translational & Translational Prof. Gaurisankar Sa, India Dr. Mohan Wani, India v Nanomedicine Research Dr. D. K. Mitra, India v Biomarkers v MetaBolomics Dr. Mahak Sharma, India v Epigenetics v Biology of Ayurveda Dr. Suvendra N. Bhattacharya, India Dr. Kalika Prasad, India v Proteomics v Genomics & Big Data Analysis Dr. -

Research Report
RESEARCH REPORT April 2018 - March 2019 Research Tree Concept note: The symbol of a tree has been used to denote research as the driving force for dynamic growth and transformational development. The interconnected branches represent the collaborative nature of all the disciplines, nurturing NU's mission of scholarly excellence. Annual Research Report (2018-19) Nirma University Sarkhej-Gandhinagar Highway, Ahmedabad- 382 481 Website: www.nirmauni.ac.in Editorial Board 1. Dr. P. N. Tekwani Director, Research and Innovation, Chairman – Editorial Board 2. Dr. Alka Mahajan Director, Institute of Technology 3. Dr. M. Mallikarjun Director, Institute of Management 4. Dr. Manjunath Ghate Director, Institute of Pharmacy 5. Dr. Sarat Dalai Director, Institute of Science 6. Dr. Purvi Pokhariyal Director, Institute of Law 7. Prof. Utpal Sharma Director, Institute of Architecture and Planning 8. Dr. Udai Paliwal Dean, Faculty of Commerce 9. Dr. R. N. Patel Additional Director, School of Engineering, Institute of Technology 10. Prof. Sangita Shroff Head, Department of Design 11. Dr. Santosh Vora Dy. Director, Research and Innovation 12. Dr. Tejal Mehta Dy. Director, Centre for Quality Assurance & Academic Development 13. Shri G. R. Nair Executive Registrar, Nirma University 14. Dr. Heena Dave Assistant Research Scientist, Directorate of Research and Innovation Member Secretary – Editorial Board 15. Ms. Ritu Agarwal Publication Officer, Nirma University Nirma University Annual Research Report (2018-19) Page i Message from the Vice President Over the years, Nirma University has made a distinct reputation for itself due to its constant efforts to stay relevant in the changing times and upgrade its curriculum and pedagogy. The University is constantly evolving as a well-known university on the international map.