Microbe Farmers: How Fermentation Artisans Are Bringing Peace to the War on Microbes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
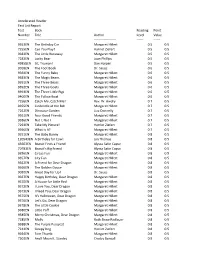
Accelerated Reader Test List Report Test Book Reading Point Number
Accelerated Reader Test List Report Test Book Reading Point Number Title Author Level Value -------- ---------------------------------- -------------------- ------ ------ 9353EN The Birthday Car Margaret Hillert 0.5 0.5 7255EN Can You Play? Harriet Ziefert 0.5 0.5 9382EN The Little Runaway Margaret Hillert 0.5 0.5 7282EN Lucky Bear Joan Phillips 0.5 0.5 49858EN Sit, Truman! Dan Harper 0.5 0.5 9018EN The Foot Book Dr. Seuss 0.6 0.5 9364EN The Funny Baby Margaret Hillert 0.6 0.5 9383EN The Magic Beans Margaret Hillert 0.6 0.5 9391EN The Three Bears Margaret Hillert 0.6 0.5 9392EN The Three Goats Margaret Hillert 0.6 0.5 9393EN The Three Little Pigs Margaret Hillert 0.6 0.5 9400EN The Yellow Boat Margaret Hillert 0.6 0.5 7256EN Catch Me, Catch Me! Rev. W. Awdry 0.7 0.5 9355EN Cinderella at the Ball Margaret Hillert 0.7 0.5 7262EN Dinosaur Garden Liza Donnelly 0.7 0.5 9361EN Four Good Friends Margaret Hillert 0.7 0.5 9386EN Not I, Not I Margaret Hillert 0.7 0.5 7293EN Take My Picture! Harriet Ziefert 0.7 0.5 9396EN What Is It? Margaret Hillert 0.7 0.5 9351EN The Baby Bunny Margaret Hillert 0.8 0.5 120543EN A Birthday for Cow! Jan Thomas 0.8 0.5 43663EN Biscuit Finds a Friend Alyssa Satin Capuc 0.8 0.5 70783EN Biscuit's Big Friend Alyssa Satin Capuc 0.8 0.5 9356EN Circus Fun Margaret Hillert 0.8 0.5 9357EN City Fun Margaret Hillert 0.8 0.5 9362EN A Friend for Dear Dragon Margaret Hillert 0.8 0.5 9366EN The Golden Goose Margaret Hillert 0.8 0.5 9020EN Great Day for Up! Dr. -

Brewer's Yeast: Genetics and Biotechnology
Applied Mycology and Biotechnology Volume 2. Agriculture and Food Production 1 @ 2002 Elsevier Science B.V. Al! rights reserved Brewer's Yeast: Genetics and Biotechnology Julio Polaina Instituto de Agroquímica y Tecnología de Alimentos, Consejo Superior de Investigaciones Científicas, Apartado de Correos 73, E46100-Burjasot (Valencia), Spain (E-Mail:jpola,[email protected]). The advance of Science in fue 19thcentury was a decisive force for fue deve10pmentand expansion of fue modero brewing industry. Correspondingly, fue brewing industry contributed important scientific achievements, such as Hansen's isolation of pure yeast cultures. Early studies on yeast were connected to fue development of different scientific disciplines such as Microbiology, Biochemistry and Genetics. An example of this connection is Winge's discovery of Mendelian inheritance in yeast. However, genetic studies with fue specific type of yeast used in brewing were hampered by fue complex constitution of this organismo The emergence of Molecular Biology allowed a precise characterization of fue brewer's yeast and fue manipulation of its properties, aimed at fue improvement of the brewing process and fue quality of fue beer. 1. INTRODUCTION The progress of chemistry, physiology and microbiology during the 19th.Century,allowed a scientific approach to brewing that caused a tremendousadvancement on fue production of beer. The precursor of such approach was the French microbiologist Louis Pasteur. At this time, fue Danish brewer Jacob Christian Jacobsen,algO founded fue Carlsberg Brewery and fue Carlsberg Laboratory. In Jacobsen'sown words, fue purpose ofthe Carlsberg Laboratory was: "By independent investigation to test the doctrines already furnished by Science and by continued studies to develop them into asfully scientij/c a basis as possible for the operation of mailing, brewing and fermentation". -

Accelerated Reader Book List 08-09
Accelerated Reader Book List 08-09 Test Number Book Title Author Reading Level Point Value -------------------------------------------------------------------------- 35821EN 100th Day Worries Margery Cuyler 3.0 0.5 107287EN 15 Minutes Steve Young 4.0 4.0 8251EN 18-Wheelers Linda Lee Maifair 5.2 1.0 661EN The 18th Emergency Betsy Byars 4.7 4.0 7351EN 20,000 Baseball Cards...Sea Jon Buller 2.5 0.5 30561EN 20,000 Leagues Under the Sea (Gr Verne/Vogel 5.2 3.0 6201EN 213 Valentines Barbara Cohen 4.0 1.0 166EN 4B Goes Wild Jamie Gilson 4.6 4.0 8252EN 4X4's and Pickups A.K. Donahue 4.2 1.0 9001EN The 500 Hats of Bartholomew Cubb Dr. Seuss 4.0 1.0 31170EN The 6th Grade Nickname Game Gordon Korman 4.3 3.0 413EN The 89th Kitten Eleanor Nilsson 4.7 2.0 71428EN 95 Pounds of Hope Gavalda/Rosner 4.3 2.0 81642EN Abduction! Peg Kehret 4.7 6.0 11151EN Abe Lincoln's Hat Martha Brenner 2.6 0.5 101EN Abel's Island William Steig 5.9 3.0 76357EN The Abernathy Boys L.J. Hunt 5.3 6.0 9751EN Abiyoyo Pete Seeger 2.2 0.5 86635EN The Abominable Snowman Doesn't R Debbie Dadey 4.0 1.0 117747EN Abracadabra! Magic with Mouse an Wong Herbert Yee 2.6 0.5 815EN Abraham Lincoln Augusta Stevenson 3.5 3.0 17651EN The Absent Author Ron Roy 3.4 1.0 10151EN Acorn to Oak Tree Oliver S. Owen 2.9 0.5 102EN Across Five Aprils Irene Hunt 6.6 10.0 7201EN Across the Stream Mirra Ginsburg 1.7 0.5 17602EN Across the Wide and Lonesome Pra Kristiana Gregory 5.5 4.0 76892EN Actual Size Steve Jenkins 2.8 0.5 86533EN Adam Canfield of the Slash Michael Winerip 5.4 9.0 118142EN Adam Canfield, -

Food: Just Grow It!
Food: Just Grow It! Developed with funding support from the Healthy Hawai`i Initiative State of Hawai`i Department of Health __________________________________________________ PROJECT LEADERS: University of Hawaii College of Tropical Agriculture and Human Resources Cooperative Extension Hawaii State Department of Education Food: Just Grow It! … a supplementary compendium of teaching-learning activities designed to enhance secondary students’ thinking and reasoning skills … __________________________________________________ University of Hawaii College of Tropical Agriculture and Human Resources Cooperative Extension Hawaii State Department of Education February 2004 FFoooodd:: JJuusstt GGrrooww IItt!! TABLE OF CONTENTS OVERVIEW: 1-6 ACTIVITIES: “Rot for Your Plot” Introduction to Theme Units 7 Creating Soil (Weathering Effects) 9 Hot Spots (Warming and Cooling) 16 Porous or Poor-Us (Soil Characteristics) 25 Taste of Dirt? (pH) 36 Dirt Rich (Soil in the Food Cycle) 45 Under-Cover Critters & Creatures (Composting) 54 Compost Cook-Off (Making Compost) 63 “Why Organic Growing?” Introduction to Theme Units 71 Malama i ka `Aina (Hawaiian Culture) 73 Victory Gardens (WW II Oral History) 83 What Goes Down Stays Around (Water Cycle) 92 OG-What? (Organic Farming Certification) 105 People’s Perceptions (Organic Farming Survey) 114 The Great Debate (Organic vs. High-Intensity) 125 WOG It! (Growing Organically) 133 “Know Your Pests” Introduction to Theme Units 141 Pest-iness (Informal Classification) 143 Least “Wanted” (Local Pest / Disease Problem) -

Beauty and the Yeast
Beauty and the Yeast Brewer’s yeast what, how and why should I care? Troels Prahl Vice President of Innovation and European Operations #2 Agenda • History of beer yeast • Yeast metabolism basics - Flavor creation • White Labs • Yeast handling -Pitching -Fermentation -Collection and reuse #3 In the beginning…… • Laboratories for commercial yeast did not exist • Brewing strains were created by brewers by: – Continuing to use strains that performed well and tasted good – Passing strains to brewer to brewer 4 The Beginning of Yeast Banking • Emil Christian Hansen 1883 – Developed pure culture techniques • Strain selection and strain storage Yeast Used in Brewing All yeast used in brewing worldwide is non-GMO Ale Yeast • Original brewing strain - Saccharomyces cerevisiae • Top ferment Lager Yeast • Warmer fermentation • Natural hybrid - temps *Saccharomyces • Wide strain variety pastorianus (Saccharomyces Other Strains carlsbergensis) • Saccharomyces uvarum • Bottom ferment • Saccharomyces bayanus • Colder fermentation temps • Saccharomyces • Limited strain variety eubayanus *Saccharomyces pastorianus = Saccharomyces cerevisiae + Saccharomyces eubayanus. 6 Predictability... 1. Reliable growth and fermentation 2. Short lag phase, normal yield 3. Suited for wort medium and conditions (pH, sugars, nutrients, temperature) 4. High attenuation 5. Low acid production 6. Desired flocculation 7. Desired aroma profile 8. Stable and robust culture 9. Safe for food production ...and Creativity! Yeast does not care about making beer, it only cares about -

Novel Non-Cerevisiae Saccharomyces Yeast Species Used in Beer and Alcoholic Beverage Fermentations
fermentation Review Novel Non-Cerevisiae Saccharomyces Yeast Species Used in Beer and Alcoholic Beverage Fermentations James Bruner * and Glen Fox * Food Science and Technology, University of California, Davis, CA 95616, USA * Correspondence: [email protected] (J.B.); [email protected] (G.F.) Received: 28 October 2020; Accepted: 22 November 2020; Published: 24 November 2020 Abstract: A great deal of research in the alcoholic beverage industry was done on non-Saccharomyces yeast strains in recent years. The increase in research interest could be attributed to the changing of consumer tastes and the search for new beer sensory experiences, as well as the rise in popularity of mixed-fermentation beers. The search for unique flavors and aromas, such as the higher alcohols and esters, polyfunctional thiols, lactones and furanones, and terpenoids that produce fruity and floral notes led to the use of non-cerevisiae Saccharomyces species in the fermentation process. Additionally, a desire to invoke new technologies and techniques for making alcoholic beverages also led to the use of new and novel yeast species. Among them, one of the most widely used non-cerevisiae strains is S. pastorianus, which was used in the production of lager beer for centuries. The goal of this review is to focus on some of the more distinct species, such as those species of Saccharomyces sensu stricto yeasts: S. kudriavzevii, S. paradoxus, S. mikatae, S. uvarum, and S. bayanus. In addition, this review discusses other Saccharomyces spp. that were used in alcoholic fermentation. Most importantly, the factors professional brewers might consider when selecting a strain of yeast for fermentation, are reviewed herein. -

How to Manage Yeast for the Home Brewery
How to Manage Yeast for the Home Brewery Dr. Douglas Gladue University of Connecticut / Plum Island Animal Disease Center BJCP National Judge How I started with yeast - Graduate work: Stony Brook University -Thesis work in the Konopka Lab - was on cell signaling in Saccharomyces cerevisiae -Homebrewer since Jan 2002 Why study yeast? • Single cell organism • One of the simplest Eukaryotic organisms – Bacteria are prokaryotic – Humans and all animals Eukaryotic • Many cellular processes are conserved in yeast and humans • About 20% of gene linked diseases in humans have a corresponding gene in yeast • Many drugs were first tested in yeast. Advantages of working in a yeast lab • Always had a supply of yeast growth media • Autoclaves for sterility • “free consumables” • Ability to isolate and freeze down any yeast strain • Could have enough yeast to pitch overnight • Yeast strains were always “Free” UCONN/ Plum Island: Use Yeast to Study Viruses Yeast and the Homebrewer • Liquid yeasts not always available or fresh • Expensive some times 1/3 of the cost of ingredients • Spontaneous brew day • Typically requires dried yeast or homebrew store visit • Starters grow more slowly • Lab optimal growth conditions • Freezing/ Storing yeasts more difficult • Most homes don’t have – a -70oC freezer (typical home freezer is -20) – Sterile working environments Overview • History of yeast • How yeast grow/ferment • Yeast terminology/choosing a yeast strain • New advances in yeast technology • Simple ways to harvest yeast • Maintaining a house yeast/reusing yeast Discovery of yeast for fermentation • 7000 years ago: Oldest records of beer • Inoculation stick (vat to vat) • Anton van Leeuwenhoek 1680 – First to observe yeast • Theodore Schwann 1837 – Determined yeast was alive (Zuckerpilz) – Latin translation: Saccharomyces • Louis Pasteur 1866 • fermentation was from living cells Photos from wikipedia Yeast and Brewing • 1876 Carlsberg established first brewing laboratory • 1883 was the first brewery using pure yeast cultures Emil Christian Hansen. -

Jacobsen and the Carlsberg Brewery in Copenhagen Fathi Habashi
Laval University From the SelectedWorks of Fathi Habashi May, 2018 Jacobsen and the Carlsberg Brewery in Copenhagen Fathi Habashi Available at: https://works.bepress.com/fathi_habashi/283/ Jacobsen and the Carlsberg Brewery in Copenhagen1 Fathi Habashi Laval University, Quebec City, Canada [email protected] ABSTRACT Scientific beer making was achieved in Copenhagen when Jacob Christian Jacobsen founded in 1876 the Carlsberg Brewery. He founded a laboratory where chemists and biochemists devised scientific methods for chemical analysis and fermentation chemistry. Through the Carlsberg Foundation, Jacobsen and his son Carl were generous philanthropists and art collectors that contributed greatly to the development of Denmark. INTRODUCTION Innovations in the brewing process came about with the introduction of the thermometer in 1760 and hydrometer in 1770, which allowed brewers to increase efficiency. Louis Pasteur's 1857 discovery of yeast's role in fermentation led to brewers developing methods to prevent the souring of beer by undesirable microorganisms. The advent of automatic bottling, commercial refrigeration, and the rise of the railroads made mass production and distribution possible. In 1876 The Danish industrialist Jacob Christian Jacobsen (1811–1887) (Figure 1) founded the Carlsberg Brewery (Figures 2-4) on the outskirts of Copenhagen and named it after his son Carl. The Carlsberg Group became a global brewer employing around 41,000 people, primarily located in Europe and Asia. The old brewery is currently open for tours. In the courtyard of the Visitors Centre is a smaller replica of the Little Mermaid Figure 1- Jacob Christian Statue (Figure 5) that Jacobsen donated Jacobsen (1811 –1887) to Copenhagen. -

Read Razorcake Issue #36 As a PDF
So Very Unsexy shove—but any more rules than that, and it’s only a matter of time that I thought of this in Gainesville, Florida at The Fest. I was in your brushstrokes of good intentions have you painted into a corner. ZRQGHU 1R ¿JKWV 1R EXOOVKLW *UHDW SHRSOH *UHDW PXVLF $Q Here’s the connection: Too often, the folks who have the most expertly planned, large event. How’d this happen? “expert without actually doing it” advice are the ones who want more I’m not deaf, but I do admit that ninety-eight percent of the time rules enforced because they don’t feel in control of the situation; ZKHQ,KHDU³+H\GXGH\RXVKRXOGBBBBBBBBB ¿OOLQWKHEODQN hell, they may even have the best of intentions. But, ultimately, they with the zine. That’d totally keep it from suckin’,” I’m no longer want to steer from the back seat—yank the wheel—want to have paying attention. I’m picturing this really cute, fuzzy otter and he’s equal time without equal effort. They have the opportunity to walk ÀRDWLQJRQKLVEDFNWU\LQJWRFUDFNRSHQWKLVFODPZLWKDURFN0DQ away from a car you built if they wreck it. ,ORYHWKDWRWWHUJHWWLQJDOO'DUZLQRQWKHVKHOO¿VKORXQJLQJLQWKH ,¶OOOHW\RXLQRQDFRXSOHRIVHFUHWV 5D]RUFDNH¶VQRWEOLQGO\ ocean, being all cute, calm, and concentrated. And by the time the groping along, bumping into things, and somehow a zine ploops out person’s done telling me what I should do—without them offering of heinies every two months. It’s a lot of very tedious, constant work to take up the task they’re suggesting—that little otter’s opened WKDWLVVRYHU\XQVH[\WKDWLW¶VERULQJWRHYHQEULHÀ\PHQWLRQZKDW up that shell; he smiles as he eats. -

Product Catalog
PRODUCT CATALOG Advancing fermentation. Cultivating community. V.10.3 What began as homebrewers searching for higher quality yeast quickly grew into a team of dedicated biochemists exploring new ways to advance brewing altogether. Today, White Labs stands at the intersection of science, education and craft. Constantly striving for perfection, and in the process continually raising the bar in the art of fermentation. Every day, we set out on a single mission: to stretch the limits of science in order to set new standards in purity and freshness. From the industry’s first pitchable liquid yeast, to a complete revolution in the way it’s propagated and packaged, our innovative spirit is tireless. Our belief is that creating the best products goes hand-in-hand with making the best use of them. This has inspired a culture of education and collaboration with brewers, distillers and winemakers the world over. At White Labs, our core business is making pitchable yeast for beer, wine, cider and spirits. We also supply third party testing, conduct educational seminars and consult on a variety of topics for these industries. It all started in 1995 with a hobby of homebrewing and the simple desire to make something better. I was lucky to befriend other homebrewers who would become some of the world’s greatest professional brewers, and they needed a better supply of yeast at a time when pure cultures were not readily available. Yeast is easy to make but very hard to make well, and any imperfections can be detected, which makes using fresh cultures extremely important. -

The Impact of Commercially Available Ale and Lager Yeasts Strains on The
bioRxiv preprint doi: https://doi.org/10.1101/2020.07.17.209171; this version posted July 18, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. ORIGINAL MANUSCRIPT The impact of commercially available ale and lager yeasts strains on the fermentative diversity of beers Diego Bonattoa,* aBrewing Yeast Research Group, Centro de Biotecnologia da UFRGS, Departamento de Biologia Molecular e Biotecnologia, Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brazil Short title: Yeast diversity in brewing *Corresponding author: Diego Bonatto Centro de Biotecnologia da UFRGS - Sala 107 Departamento de Biologia Molecular e Biotecnologia Universidade Federal do Rio Grande do Sul – UFRGS Avenida Bento Gonçalves 9500 - Prédio 43421 Caixa Postal 15005 Porto Alegre – Rio Grande do Sul BRAZIL 91509-900 Phone: (+55 51) 3308-7765 E-mail: [email protected] Contract/grant sponsor: CNPq 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.07.17.209171; this version posted July 18, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Yeasts from the species Saccharomyces cerevisiae (ale yeast) and Saccharomyces pastorianus (lager yeast) are the main component of beer fermentation. It is known that different beer categories depends on the use of specific ale or lager strains, where the yeast will imprint its distinctive fermentative profile to the beer. Despite this, there is no studies reporting about how diverse, rich, and homogeneous are beer categories in terms of commercially available brewing yeast strains. -
![[Name and Address (Press F11 to Jump to the Next Field)]](https://docslib.b-cdn.net/cover/0605/name-and-address-press-f11-to-jump-to-the-next-field-3780605.webp)
[Name and Address (Press F11 to Jump to the Next Field)]
Carlsberg A/S 100 Ny Carlsberg Vej Tel +45 3327 3300 1799 Copenhagen V [email protected] Denmark CVR.nr. 61056416 Press release 16/2016 Copenhagen, 29 November, 2016 Page 1 of 2 Four awards at Carlsberg’s “Science to business” forum Carlsberg Foundation and the Carlsberg Research Laboratory hosted its annual awards celebration today. A record number of four awards were handed out this year to commemorate the Laboratory’s 140th anniversary. The Carlsberg Forum series reflects the strong link between science and business. Each year, the Kaj Linderstrøm-Lang awards are given to prominent scientists for their achievements within biochemistry or physiology, the fields of science in which Kaj Linderstrøm-Lang, a professor at Carlsberg Research Laboratory in the period 1939-1959, distinguished himself as a pioneer. This year, Professor Henrik V. Scheller, Joint BioEnergy Institute, Lawrence Berkeley National Laboratory, USA and Professor Geoff Fincher, School of Agriculture, Food & Wine, The University of Adelaide, Australia received this year’s Kaj Linderstrøm-Lang Prize as an acknowledgement of their outstanding achievements on identifying and characterizing enzymes involved in synthesis and modification of the plant cell wall. The third Kaj Linderstrøm-Lang Prize was awarded to Professor Richard Henderson, MRC Laboratory of Molecular Biology, Cambridge, UK for his pioneering work towards obtaining high resolution atomic structures of membrane proteins and membrane protein complexes by electron cryomicroscopy. The prize is a plated gold medal and a financial personal award of DKK 40.000. Finally, the Emil Chr. Hansen Golden Medal was awarded to Jef Boeke, Director, Institute for Systems Genetics at New York University, for his seminal contributions to yeast genome scrambling and the design of synthetic yeast.