3Rd Quarter 2002 Medicare B Update
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Acute Limb Ischemia Secondary to Myositis- Induced Compartment Syndrome in a Patient with Human Immunodeficiency Virus Infection
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Acute limb ischemia secondary to myositis- induced compartment syndrome in a patient with human immunodeficiency virus infection Russell Lam, MD, Peter H. Lin, MD, Suresh Alankar, MD, Qizhi Yao, MD, PhD, Ruth L. Bush, MD, Changyi Chen, MD, PhD, and Alan B. Lumsden, MD, Houston, Tex Myositis, while uncommon, develops more frequently in patients with human immunodeficiency virus infection. We report a case of acute lower leg ischemia caused by myositis in such a patient. Urgent four-compartment fasciotomy of the lower leg was performed, which decompressed the compartmental hypertension and reversed the arterial ischemia. This case underscores the importance of recognizing compartment syndrome as a cause of acute limb ischemia. (J Vasc Surg 2003;37:1103-5.) Compartment syndrome results from elevated pressure compartment was firm and tender. Additional pertinent laboratory within an enclosed fascial space, which can occur after studies revealed creatine phosphokinase level of 53,350 U/L; fracture, soft tissue injury, or reperfusion after arterial isch- serum creatinine concentration had increased to 3.5 mg/dL, and emia.1 Other less common causes of compartment syn- WBC count had increased to 18,000 cells/mm3. Venous duplex drome include prolonged limb compression, burns, and scans showed no evidence of deep venous thrombosis in the right extreme exertion.1 Soft tissue infection in the form of lower leg. Pressure was measured in all four compartments of the myositis is a rare cause of compartment syndrome. We right calf and ranged from 55 to 65 mm Hg. -

White Collar Crime by Health Care Providers Pamela H
NORTH CAROLINA LAW REVIEW Volume 67 | Number 4 Article 7 4-1-1989 Fraud by Fright: White Collar Crime by Health Care Providers Pamela H. Bucy Follow this and additional works at: http://scholarship.law.unc.edu/nclr Part of the Law Commons Recommended Citation Pamela H. Bucy, Fraud by Fright: White Collar Crime by Health Care Providers, 67 N.C. L. Rev. 855 (1989). Available at: http://scholarship.law.unc.edu/nclr/vol67/iss4/7 This Article is brought to you for free and open access by Carolina Law Scholarship Repository. It has been accepted for inclusion in North Carolina Law Review by an authorized administrator of Carolina Law Scholarship Repository. For more information, please contact [email protected]. FRAUD BY FRIGHT: WHITE COLLAR CRIME BY HEALTH CARE PROVIDERSt PAMELA H. Bucyt Fraudby health care providers is one of the most deleterious of all white collar crimes. It is also one of the most difficult to prosecute. In her Article, ProfessorBucy comparesfraud by health care providers with other types of white collar crime and analyzes the theories offraud his- torically used to prosecute health careproviders. She concludes that the strongest theory--prosecutionfor providing unnecessary or substandard health care-is the theory that has been used the least. ProfessorBucy suggests ways for prosecutors to use this theory more often and more effectively in order to combat a problem that ravishes human dignity and personal health as well as the nationalpocketbook "I will apply measures for the benefit of the sick according to my ability and judgment; I will keep them from harm and injustice." Portion of Oath of Hippocrates, Sixth Century B.C.- First Century A.D.; currently administered by many medical schools to graduating medical students.1 "[I c]ould make a million dollars out of the suckers ..... -

Evaluation of Suspected Malignant Hyperthermia Events During Anesthesia Frank Schuster*, Stephan Johannsen, Daniel Schneiderbanger and Norbert Roewer
Schuster et al. BMC Anesthesiology 2013, 13:24 http://www.biomedcentral.com/1471-2253/13/24 RESEARCH ARTICLE Open Access Evaluation of suspected malignant hyperthermia events during anesthesia Frank Schuster*, Stephan Johannsen, Daniel Schneiderbanger and Norbert Roewer Abstract Background: Malignant hyperthermia (MH), a metabolic myopathy triggered by volatile anesthetics and depolarizing muscle relaxants, is a potentially lethal complication of general anesthesia in susceptible patients. The implementation of modern inhalation anesthetics that research indicates as less potent trigger substances and the recommended limitations of succinylcholine use, suggests there may be considerable decline of fulminant MH cases. In the presented study, the authors analyzed suspected MH episodes during general anesthesia of patients that were referred to the Wuerzburg MH unit between 2007 and 2011, assuming that MH is still a relevant anesthetic problem in our days. Methods: With approval of the local ethics committee data of patients that underwent muscle biopsy and in vitro contracture test (IVCT) between 2007 and 2011 were analyzed. Only patients with a history of suspected MH crisis were included in the study. The incidents were evaluated retrospectively using anesthetic documentation and medical records. Results: Between 2007 and 2011 a total of 124 patients were tested. 19 of them were referred because of suspected MH events; 7 patients were diagnosed MH-susceptible, 4 MH-equivocal and 8 MH-non-susceptible by IVCT. In a majority of cases masseter spasm after succinylcholine had been the primary symptom. Cardiac arrhythmias and hypercapnia frequently occurred early in the course of events. Interestingly, dantrolene treatment was initiated in a few cases only. -

The Rigid Spine Syndrome-A Myopathy of Uncertain Nosological Position
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.48.9.887 on 1 September 1985. Downloaded from Journal ofNeurology, Neurosurgery, and Psychiatry 1985;48:887-893 The rigid spine syndrome-a myopathy of uncertain nosological position W POEWE,* H WILLEIT,* E SLUGA,t U MAYR* From the University Clinic for Neurology, Innsbruck, * and the Neurological Instiute ofthe University of Vienna, Vienna,t Austria SUMMARY Four patients meeting the clinical criteria of the rigid spine syndrome are presented; they are one girl with a positive family history and three boys. Clinical and histological findings are discussed in relation to the 14 cases of rigid spine syndrome reported in the literature. The delineations of the syndrome from other benign myopathies with early contractures are discussed suggesting that the rigid spine syndrome probably does not represent a single nosological entity. In 1965 Dubowitz' drew attention to a muscular Case reports disorder resembling muscular dystrophy at the time guest. Protected by copyright. of its onset in infancy but of benign and non progres- Since 1978 the authors have had the opportunity to sive nature with the development of only mild examine clinically, electrophysiologically and by muscle weakness. The central clinical feature in this condi- biopsy four cases of a muscle disorder fulfilling the clinical criteria of rigid spine syndrome as described by tion is marked limitation of flexion of the cervical Dubowitz.' The patients were three males and one and dorsolumbar spine with the development of female, whose sister is thought to suffer from the same scoliosis and associated contractures of other joints, disorder. -

Fostering Innovation to Fight Waste, Fraud, and Abuse in Health Care
FOSTERING INNOVATION TO FIGHT WASTE, FRAUD, AND ABUSE IN HEALTH CARE HEARING BEFORE THE SUBCOMMITTEE ON HEALTH OF THE COMMITTEE ON ENERGY AND COMMERCE HOUSE OF REPRESENTATIVES ONE HUNDRED THIRTEENTH CONGRESS FIRST SESSION FEBRUARY 27, 2013 Serial No. 113–10 ( Printed for the use of the Committee on Energy and Commerce energycommerce.house.gov U.S. GOVERNMENT PRINTING OFFICE 80–160 WASHINGTON : 2013 For sale by the Superintendent of Documents, U.S. Government Printing Office Internet: bookstore.gpo.gov Phone: toll free (866) 512–1800; DC area (202) 512–1800 Fax: (202) 512–2104 Mail: Stop IDCC, Washington, DC 20402–0001 VerDate Nov 24 2008 12:32 May 15, 2013 Jkt 037690 PO 00000 Frm 00001 Fmt 5011 Sfmt 5011 F:\MY DOCS\HEARINGS 113\113-10 CHRIS COMMITTEE ON ENERGY AND COMMERCE FRED UPTON, Michigan Chairman RALPH M. HALL, Texas HENRY A. WAXMAN, California JOE BARTON, Texas Ranking Member Chairman Emeritus JOHN D. DINGELL, Michigan ED WHITFIELD, Kentucky Chairman Emeritus JOHN SHIMKUS, Illinois EDWARD J. MARKEY, Massachusetts JOSEPH R. PITTS, Pennsylvania FRANK PALLONE, JR., New Jersey GREG WALDEN, Oregon BOBBY L. RUSH, Illinois LEE TERRY, Nebraska ANNA G. ESHOO, California MIKE ROGERS, Michigan ELIOT L. ENGEL, New York TIM MURPHY, Pennsylvania GENE GREEN, Texas MICHAEL C. BURGESS, Texas DIANA DEGETTE, Colorado MARSHA BLACKBURN, Tennessee LOIS CAPPS, California Vice Chairman MICHAEL F. DOYLE, Pennsylvania PHIL GINGREY, Georgia JANICE D. SCHAKOWSKY, Illinois STEVE SCALISE, Louisiana ANTHONY D. WEINER, New York ROBERT E. LATTA, Ohio JIM MATHESON, Utah CATHY MCMORRIS RODGERS, Washington G.K. BUTTERFIELD, North Carolina GREGG HARPER, Mississippi JOHN BARROW, Georgia LEONARD LANCE, New Jersey DORIS O. -

Entire Issue (PDF)
E PL UR UM IB N U U S Congressional Record United States th of America PROCEEDINGS AND DEBATES OF THE 114 CONGRESS, SECOND SESSION Vol. 162 WASHINGTON, THURSDAY, FEBRUARY 4, 2016 No. 21 House of Representatives The House met at 10 a.m. and was last day’s proceedings and announces Red, White, and Blue’s great work in called to order by the Speaker pro tem- to the House his approval thereof. enriching the lives of veterans in need. pore (Mr. MOONEY of West Virginia). Pursuant to clause 1, rule I, the Jour- I had the great privilege of knowing f nal stands approved. Ted personally and was inspired by his kindness, his humor, and his love for DESIGNATION OF THE SPEAKER f his family and country. Ted will al- PRO TEMPORE PLEDGE OF ALLEGIANCE ways be remembered as an honorable The SPEAKER pro tempore laid be- The SPEAKER pro tempore. Will the young man who touched many lives, fore the House the following commu- gentlewoman from New York (Ms. having a lasting positive impact on all nication from the Speaker: STEFANIK) come forward and lead the who knew and loved him. WASHINGTON, DC, House in the Pledge of Allegiance. May his name forever be remembered February 4, 2016. Ms. STEFANIK led the Pledge of Al- in the CONGRESSIONAL RECORD and in I hereby appoint the Honorable ALEXANDER legiance as follows: the great United States of America. X. MOONEY to act as Speaker pro tempore on this day. I pledge allegiance to the Flag of the United States of America, and to the Repub- f PAUL D. -

Medicare Fraud and Improper Billing
Medicare Fraud and Improper Billing ISSUE BRIEF • July 2018 Part I. Medicare Fraud Prevention Micki Nozaki, Director, California Health Advocates, Senior Medicare Patrol California Health Advocates Founded in 1997, California Health Advocates (CHA) is the leading Medicare advocacy and education non-profit in California. We advocate on behalf of Medicare beneficiaries and their families; conduct public policy research to support improved rights and protections for Medicare beneficiaries; and provide accurate and up-to-date Medicare information for both Medicare beneficiaries and their families as well as the advocates and providers who serve them. Senior Medicare Patrol The California Senior Medicare Patrol (SMP) is a project under CHA. We empower and assist Medicare beneficiaries, their families, and caregivers to prevent, detect, and report healthcare fraud, errors and abuse through: (1) Education: SMPs give presentations to groups, exhibit at events and work one-on-one with Medicare beneficiaries; (2) Counseling: SMPs work to protect older adults’ health, finances and medical identity while saving precious Medicare dollars; and (3) Assisting Medicare beneficiaries, caregivers and family members when they bring their concerns or complaints to the SMP. We determine whether fraud, errors, or abuse are suspected. When fraud or abuse is assumed, we make referrals to the appropriate state and federal agencies for further investigation. There are 54 Senior Medicare Patrol (SMP) programs throughout the country. SMPs are grant-funded projects of the federal U.S. Department of Health and Human Services, Administration for Community Living. Key Lessons 1. The Medicare Trust Fund loses $60–$90 billion every year to fraud, errors, and abuse.Although the exact figure is impossible to measure, the U.S. -

Healthcare Fraud & Abuse Review 2017
HEALTHCARE FRAUD & ABUSE REVIEW 2017 i | BASS, BERRY & SIMS HEALTHCARE FRAUD & ABUSE REVIEW 2017 1. A LOOK BACK…A LOOK AHEAD 4. NOTEWORTHY SETTLEMENTS 7. ISSUES TO WATCH 12. FALSE CLAIMS ACT UPDATE 37. STARK LAW/ANTI-KICKBACK STATUTE 40. PHARMACEUTICAL AND MEDICAL DEVICE DEVELOPMENTS 42. APPENDIX – 2017 NOTABLE SETTLEMENTS Hospitals and Health Systems Managed Care/Insurance Hospice Laboratory, Pathology, Radiology and Diagnostics Home Health Specialty Care and Other Provider Entities SNFs and Nursing Homes Individual Providers Pharmaceutical and Device Miscellaneous/Non-Providers Pharmacy Services 70. ABOUT BASS, BERRY & SIMS General (HHS-OIG), along with federal and state law enforcement entities, including 30 Medicaid Fraud Control Units, charged more than 400 defendants, including 115 healthcare professionals, A LOOK BACK… in 41 federal districts for allegedly participating in fraudulent healthcare arrangements resulting in over $1.3 billion in false claims.6 A LOOK AHEAD Occurring in the context of combatting the opioid crisis, the takedown focused on individuals allegedly involved in fraudulent billing of Medicare, Medicaid and TRICARE for medically While the uncertainty associated with legislative efforts to repeal unnecessary prescription and compounded drugs that were not actually purchased or distributed to patients covered by a federal healthcare program. In total, more than 120 the Patient Protection and Affordable Care Act (PPACA) dominated defendants, including physicians, were charged in connection with prescribing and distributing most of the headlines for the healthcare industry last year, it was opioids and narcotics. Nearly 300 individuals—including physicians, nurses and pharmacists— mostly business as usual for the government’s healthcare fraud received exclusion notices from HHS-OIG barring future participation in federal healthcare 7 enforcement efforts. -
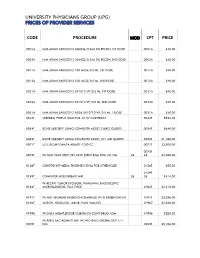
Code Procedure Cpt Price University Physicians Group
UNIVERSITY PHYSICIANS GROUP (UPG) PRICES OF PROVIDER SERVICES CODE PROCEDURE MOD CPT PRICE 0001A IMM ADMN SARSCOV2 30MCG/0.3ML DIL RECON 1ST DOSE 0001A $40.00 0002A IMM ADMN SARSCOV2 30MCG/0.3ML DIL RECON 2ND DOSE 0002A $40.00 0011A IMM ADMN SARSCOV2 100 MCG/0.5 ML 1ST DOSE 0011A $40.00 0012A IMM ADMN SARSCOV2 100 MCG/0.5 ML 2ND DOSE 0012A $40.00 0021A IMM ADMN SARSCOV2 5X1010 VP/0.5 ML 1ST DOSE 0021A $40.00 0022A IMM ADMN SARSCOV2 5X1010 VP/0.5 ML 2ND DOSE 0022A $40.00 0031A IMM ADMN SARSCOV2 AD26 5X10^10 VP/0.5 ML 1 DOSE 0031A $40.00 0042T CEREBRAL PERFUS ANALYSIS, CT W/CONTRAST 0042T $954.00 0054T BONE SURGERY USING COMPUTER ASSIST, FLURO GUIDED 0054T $640.00 0055T BONE SURGERY USING COMPUTER ASSIST, CT/ MRI GUIDED 0055T $1,188.00 0071T U/S LEIOMYOMATA ABLATE <200 CC 0071T $2,500.00 0075T 0075T PR TCAT PLMT XTRC VRT CRTD STENT RS&I PRQ 1ST VSL 26 26 $2,208.00 0126T CAROTID INT-MEDIA THICKNESS EVAL FOR ATHERSCLER 0126T $55.00 0159T 0159T COMPUTER AIDED BREAST MRI 26 26 $314.00 PR RECTAL TUMOR EXCISION, TRANSANAL ENDOSCOPIC 0184T MICROSURGICAL, FULL THICK 0184T $2,315.00 0191T PR ANT SEGMENT INSERTION DRAINAGE W/O RESERVOIR INT 0191T $2,396.00 01967 ANESTH, NEURAXIAL LABOR, PLAN VAG DEL 01967 $2,500.00 01996 PR DAILY MGMT,EPIDUR/SUBARACH CONT DRUG ADM 01996 $285.00 PR PERQ SAC AGMNTJ UNI W/WO BALO/MCHNL DEV 1/> 0200T NDL 0200T $5,106.00 PR PERQ SAC AGMNTJ BI W/WO BALO/MCHNL DEV 2/> 0201T NDLS 0201T $9,446.00 PR INJECT PLATELET RICH PLASMA W/IMG 0232T HARVEST/PREPARATOIN 0232T $1,509.00 0234T PR TRANSLUMINAL PERIPHERAL ATHERECTOMY, RENAL -

Compartment Syndrome
Rowan University Rowan Digital Works Stratford Campus Research Day 23rd Annual Research Day May 2nd, 12:00 AM A Case of Atraumatic Posterior Thigh Compartment Syndrome Nailah Mubin Rowan University Brian Katt M.D. Rothman Institute Follow this and additional works at: https://rdw.rowan.edu/stratford_research_day Part of the Cardiovascular Diseases Commons, Hemic and Lymphatic Diseases Commons, Nephrology Commons, Orthopedics Commons, Pathological Conditions, Signs and Symptoms Commons, and the Surgery Commons Let us know how access to this document benefits ouy - share your thoughts on our feedback form. Mubin, Nailah and Katt, Brian M.D., "A Case of Atraumatic Posterior Thigh Compartment Syndrome" (2019). Stratford Campus Research Day. 47. https://rdw.rowan.edu/stratford_research_day/2019/may2/47 This Poster is brought to you for free and open access by the Conferences, Events, and Symposia at Rowan Digital Works. It has been accepted for inclusion in Stratford Campus Research Day by an authorized administrator of Rowan Digital Works. A Case of Atraumatic Posterior Thigh Compartment Syndrome Nailah Mubin OMS-III1, Brian Katt MD2 1Rowan University School of Osteopathic Medicine, 2Brielle Orthopedics at Rothman Institute Introduction Hospital Course Compartment syndrome (CS): intra-compartmental pressures exceed Day 1 4:40am Day 1 5pm Day 4 Day 5 Day 9 Day 12 Day 16 Day 18 Day 19 to a point where arterial, venous and lymphatic circulation of local 1 tissues, muscles and nerves is compromised ER Admit Fasciotomy Cr=7.54 First Closure Pt reports Complete Cr=5.25 Cr=4.09 Discharge 2 • Most common after a traumatic injury . Usually occurs in the leg Dialysis Initiated Attempt increased sensation Closure Cleared by Nephro or forearm and less commonly in the thigh3 • Thigh compartment syndrome (TCS) is rare due to its larger size Surgical Intervention Discussion and more compliant borders. -

Covid Fraud Tracker
Subject name(s) Date Law Enforcement Alleged Conduct Summary Relevant statute(s) State Court Type of Action Individual subject? Corporate subject? Follow-on action? Matter Number Dates of unlawful Penalties Press Release Petition/ Agreement/J Announced Agency conduct (civil/criminal fines, Complaint/ udgment incarceration) Indictment Grubhub Holdings Inc. 7/29/2021 Massachusetts AG Consumer fraud Grubhub Holdings Inc. is accused of violating a provision of Massachusetts's economic G. L. c. 93A Massachusetts D. Mass. Civil No Yes No https://www.mas development legislation, which prohibited Grubhub and other third party delivery service enforcement s.gov/news/ag- platforms from charging fees to restaurants that exceed 15 percent of an order's menu healey-sues- price. The fee cap came into effect on January 14, 2021 and remained in place until grubhub-for- Governor Baker lifted the state of emergency in Massachusetts on June 15, 2021. charging- restaurants- illegally-high-fees- during-covid-19- public-health- emergency Dinesh Sah 7/28/2021 USDOJ PPP fraud Dinesh Sah pleaded guilty to wire-fraud and money laundering for submitting 15 fraudulent 18 U.S.C. §§ 1343, 1957 Texas W.D. Tex. Criminal Yes No No 3:20-cr-00484 Incarceration; https://www.justi applications that sought $24.8 million in Paycheck Protection Program ("PPP") loans. Sah enforcement Restitution ce.gov/opa/pr/te filed the claims under the names of various businesses that he owned or controlled, xas-man- claiming these businesses had numerous employees and hundreds of thousands of dollars sentenced-24- in payroll expenses; in reality, no business had employees or paid wages consistent with the million-covid-19- amounts claimed in the PPP applications. -

Protecting Yourself & Medicare from Fraud
CENTERS for MEDICARE & MEDICAID SERVICES Protecting Yourself & Medicare from Fraud This booklet explains: ■ How to protect yourself and Medicare from fraud ■ How to identify and report billing errors and concerns ■ What to do if you suspect Medicare fraud ■ How to protect your personal information Table of contents 4 Introduction 5 How to spot & report Medicare fraud 10 Protect yourself from identity theft 10 Protect yourself when dealing with private companies who offer Medicare plans 13 Additional fraud resources 14 Tips to help prevent Medicare fraud The information in this booklet describes the Medicare program at the time this booklet was printed. Changes may occur after printing. Visit Medicare.gov, or call 1-800-MEDICARE (1-800-633-4227) to get the most current information. TTY users should call 1-877-486-2048. 3 Introduction Most doctors, health care providers, suppliers, and private companies who work with Medicare are honest, however, some aren’t. Individuals, companies, or groups can commit fraud. One example of Medicare fraud is when Medicare is billed for services or supplies that you never got. Medicare fraud wastes a lot of money each year. Fraud results in higher health care costs and taxes for everyone. Medicare is working to find and prevent fraud and abuse. We’re working more closely with health care providers and improving the way we review Medicare claims for possible billing fraud. Read this booklet to learn how you can help fight and protect yourself from fraud. 4 How to spot & report Medicare fraud Protect yourself and Medicare against fraud by reviewing your Medicare claims for errors, looking for other types of fraud, and reporting anything suspicious to Medicare.