344157-Eng.Pdf (1.882Mb)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nigeria's Constitution of 1999
PDF generated: 26 Aug 2021, 16:42 constituteproject.org Nigeria's Constitution of 1999 This complete constitution has been generated from excerpts of texts from the repository of the Comparative Constitutions Project, and distributed on constituteproject.org. constituteproject.org PDF generated: 26 Aug 2021, 16:42 Table of contents Preamble . 5 Chapter I: General Provisions . 5 Part I: Federal Republic of Nigeria . 5 Part II: Powers of the Federal Republic of Nigeria . 6 Chapter II: Fundamental Objectives and Directive Principles of State Policy . 13 Chapter III: Citizenship . 17 Chapter IV: Fundamental Rights . 20 Chapter V: The Legislature . 28 Part I: National Assembly . 28 A. Composition and Staff of National Assembly . 28 B. Procedure for Summoning and Dissolution of National Assembly . 29 C. Qualifications for Membership of National Assembly and Right of Attendance . 32 D. Elections to National Assembly . 35 E. Powers and Control over Public Funds . 36 Part II: House of Assembly of a State . 40 A. Composition and Staff of House of Assembly . 40 B. Procedure for Summoning and Dissolution of House of Assembly . 41 C. Qualification for Membership of House of Assembly and Right of Attendance . 43 D. Elections to a House of Assembly . 45 E. Powers and Control over Public Funds . 47 Chapter VI: The Executive . 50 Part I: Federal Executive . 50 A. The President of the Federation . 50 B. Establishment of Certain Federal Executive Bodies . 58 C. Public Revenue . 61 D. The Public Service of the Federation . 63 Part II: State Executive . 65 A. Governor of a State . 65 B. Establishment of Certain State Executive Bodies . -

PSWG Actors Oct 2016
protectionsector COMPLETED AND W O R K I N G G R O U P NIGERIA: PROTECTION ACTORS ON-GOING ACTIVITIES N I G E R I A Agencies with registered projects in OCHA Online Project Systems (OPS) JAN - OCT 2016 COOPI (Cooperazione Internazionale) DRC (Danish Refugee Council) IOM (International Organization for Migration) POPULATION POPULATION POPULATION REACHED 3,168 REACHED 13,363 REACHED 92,911 IMPLEMENTING PARTNERS IMPLEMENTING PARTNERS IMPLEMENTING PARTNERS YOBE BORNO Direct Implementation YOBE BORNO Direct Implementation YOBE BORNO Direct Implementation 3,168 10,988 66,908 JERE DIKWA MAIDUGURI 28 MAIDUGURI DAMATURU DAMATURU POTISKUM KONDUGA BAMA FIKA GWOZA BENEFICIARIES PER ACTIVITY CHIBOK GOMBE GOMBE MICHIKA GOMBE MUBI 2 Case Referrals NORTH GIRERI GIRERI BENEFICIARIES PER ACTIVITY 54 Capacity Building BENEFICIARIES PER ACTIVITY Unaccompanied and ADAMAWA 947 Multiple Needs ADAMAWA ADAMAWA 2 63 Livelihood Separated Children YOLA YOLA SOUTH NORTHYOLA Unaccompanied and YOLA Protection SOUTH 24 Multiple Needs 2,221 NORTH 82 Separated Children Mainstreaming FUFORE 2,375 25,975 175 Case Referrals 176 Awareness Raising / Sensitization 293 Capacity Building 271 Material Protection Assistance Psychosocial Distress Identification of 92,417 and Mental Disorder 3 6 1,727 Vulnerable Individuals 12 LOCAL GOVERNMENT LOCAL GOVERNMENT LOCAL GOVERNMENT UNIT COVERED UNIT COVERED 10,988 Dangers and Injuries UNIT COVERED NRC IRC (International Rescue Committee) NRC (Norwegian Refugee Council) Mercy Corps POPULATION POPULATION POPULATION REACHED 165,191 REACHED -

Nigeria National Emergency Action Plan – January 2017
NATIONAL PRIMARY HEALTH CARE DEVELOPMENT AGENCY 2017 NIGERIA POLIO ERADICATION EMERGENCY PLAN January 2017, Abuja NPHCDA Plot 681/682 Port Harcourt Crescent Off Gimbiya street, off Ahmadu Bello Way Garki Area 11 Abuja 1 Abbreviations AFP Acute Flaccid Paralysis AVADAR Auto-Visual AFP detection and Reporting. bOPV Bivalent oral polio vaccine BMGF Bill and Melinda Gates Foundation CDC Centers for Disease Control and Prevention CJTF Civilian Joint Task Force cVDPV Circulating Vaccine Derived Poliovirus DOPV Directly observed polio vaccination EOC Emergency Operations Centre ERC Expert Review Committee of Polio Eradication and Routine Immunization EPI Expanded Programme on Immunization FCT Federal Capital Territory FMOH Federal Ministry of Health FOMWAN Federation of Muslim Women Associations in Nigeria FRR Financial Resources Requirements GAVI Global Alliance of Vaccines and Immunization ICC Inter-agency Coordination Committee IDPs Internally displaced populations IPC Inter-personal Communication IPDs Immunization Plus Days IMB Independent Monitoring Board LGA Local Government Area LQAS Lot quality assurance sampling mOPV Monovalent oral polio vaccine NCC National Certification Committee NICS National Immunization Coverage Survey NIFAA Nigeria Interfaith Action Association NPEEP National Polio Eradication Emergency Plan NTL Northern Traditional Leaders Committee on Primary Health Care Delivery NPHCDA National Primary Health Care Development Agency OPV Oral polio vaccine PEI Polio Eradication Initiative PTFoPE Presidential Task Force on Polio Eradication RES Reaching Every Settlement RI Routine Immunization SIAs Supplemental Immunization Activities STF State Task Force on Immunization UNICEF United Nations Children’s Fund VCM Volunteer Community Mobilizer VDPV2 Vaccine derived polio virus type 2 WHO World Health Organization WPV Wild polio virus 2 CONTENTS Executive Summary ………………………………………………………………………………………………………… 4 1.0 Introduction and context of the programme ……………………………………………………………. -

PSWG Actors Nov 2016
protectionsector COMPLETED AND W O R K I N G G R O U P NIGERIA: PROTECTION ACTORS ON-GOING ACTIVITIES N I G E R I A Agencies with registered projects in OCHA Online Project Systems (OPS) JAN - NOV 2016 COOPI (Cooperazione Internazionale) DRC (Danish Refugee Council) IOM (International Organization for Migration) POPULATION POPULATION POPULATION REACHED 3,305 REACHED 14,505 REACHED 112,221 IMPLEMENTING PARTNERS IMPLEMENTING PARTNERS IMPLEMENTING PARTNERS YOBE BORNO Direct Implementation YOBE BORNO Direct Implementation YOBE BORNO Direct Implementation 3,305 11,088 82,312 JERE DIKWA MAIDUGURI 68 MAIDUGURI DAMATURU DAMATURU POTISKUM KONDUGA BAMA FIKA GWOZA BENEFICIARIES PER ACTIVITY CHIBOK GOMBE GOMBE MICHIKA GOMBE MUBI NORTH GIRERI 2 Case Referrals GIRERI BENEFICIARIES PER ACTIVITY BENEFICIARIES PER ACTIVITY 32 Livelihood Unaccompanied and ADAMAWA 947 Multiple Needs ADAMAWA ADAMAWA 2 Protection Separated Children 28 YOLA Mainstreaming YOLA SOUTH NORTHYOLA Unaccompanied and YOLA SOUTH Multiple Needs 2,358 NORTH 24 Separated Children 248 Capacity Building FUFORE 3,417 29,841 175 Case Referrals 381 Awareness Raising / Sensitization 333 Capacity Building Identification of 388 Vulnerable Individuals 111,687 Psychosocial Distress 1,267 Material Protection and Mental Disorder 3 6 Assistance 12 LOCAL GOVERNMENT LOCAL GOVERNMENT LOCAL GOVERNMENT UNIT COVERED UNIT COVERED 10,988 Dangers and Injuries UNIT COVERED NRC IRC (International Rescue Committee) NRC (Norwegian Refugee Council) Mercy Corps POPULATION POPULATION POPULATION REACHED 332,790 REACHED -

The State Independent Electoral Commissions in Nigeria: a Study of Bauchi, Edo, Imo, Kaduna, Lagos and Plateau States
The State Independent Electoral Commissions in Nigeria: A Study of Bauchi, Edo, Imo, Kaduna, Lagos and Plateau States Edited by Massoud Omar 0 Contributors Musa Abutudu Associate Professor, Department of Political Science. University of Benin. Edo State, Nigeria. Chijioke K. Iwuamadi Research Fellow, Institute for Development Studies University of Nigeria. Enugu State, Nigeria. Massoud Omar Department of Local Government Studies Ahmadu Bello University. Zaria, Kaduna State. F. Adeleke Faculty of Law, Lagos State University. Lagos State, Nigeria. Habu Galadima Department of Political Science, Bayero University, P.M.B. 3011, Kano-Nigeria Dung Pam Sha Department of Political Science, 1 University of Jos. Plateau State. 2 Table of Contents Introduction 4-10 Chapter I Bauchi State Independent Electoral Commission Habu Galadima and Aisha Omar 7-61 Chapter II The Edo State Independent Electoral Commission Musa Abutudu 62-97 Chapter III The Imo State Independent Electoral Commission (SIEC) Chijioke K. Iwuamadi 98- 135 Chapter IV The Kaduna State Independent Electoral Commission Massoud Omar 136-159 Chapter V Lagos State State Independent Electoral Commission in F.A.R Adeleke 156-191 Chapter VI The Plateau State Independent Electoral Commission: Dung Pam Sha 192-240 Conclusion 241-242 3 List of Tables and Figures Table 1.1 State of Residence Table 1.2 Local Government Area Table 1.3 Gender Table 1.4 Age Table 1.5 Marital Status Table 1.6 Occupation Table 1.7 Awareness of SIEC’s conduct of Local Government Elections Table 1.8 Number of times Respondents -

A Study of Violence-Related Deaths in Nafada Local Government Area Of
# Makai DANIEL http://www.ifra-nigeria.org/IMG/pdf/violence-related-deaths-gombe-jigawa-state-nigeria.pdf A Study of Violence-Related Deaths in Nafada Local Government Area of Gombe State and Auyo, Gagarawa, Gumel, Gwiwa, Kaugama and Yankwasi Local Government Areas of Jigawa State (2006-2014) IFRA-Nigeria working papers series, n°46 20/01/2015 The ‘Invisible Violence’ Project Based in the premises of the French Institute for Research in Africa on the campus of the University of Ibadan, Nigeria Watch is a database project that has monitored fatal incidents and human security in Nigeria since 1 June 2006. The database compiles violent deaths on a daily basis, including fatalities resulting from accidents. It relies on a thorough reading of the Nigerian press (15 dailies & weeklies) and reports from human rights organisations. The two main objectives are to identify dangerous areas and assess the evolution of violence in the country. However, violence is not always reported by the media, especially in remote rural areas that are difficult to access. Hence, in the last 8 years, Nigeria Watch has not recorded any report of fatal incidents in some of the 774 Local Government Areas (LGAs) of the Nigerian Federation. There are two possibilities: either these places were very peaceful, or they were not covered by the media. This series of surveys thus investigates ‘invisible’ violence. By 1 November 2014, there were still 23 LGAs with no report of fatal incidents in the Nigeria Watch database: Udung Uko and Urue-Offong/Oruko (Akwa Ibom), Kwaya Kusar (Borno), Nafada (Gombe), Auyo, Gagarawa, Kaugama and Yankwashi (Jigawa), Ingawa and Matazu (Katsina), Sakaba (Kebbi), Bassa, Igalamela- Odolu and Mopa-Muro (Kogi), Toto (Nassarawa), Ifedayo (Osun), Gudu and Gwadabaw (Sokoto), Ussa (Taraba), and Karasuwa, Machina, Nguru and Yunusari (Yobe). -

States and Lcdas Codes.Cdr
PFA CODES 28 UKANEFUN KPK AK 6 CHIBOK CBK BO 8 ETSAKO-EAST AGD ED 20 ONUIMO KWE IM 32 RIMIN-GADO RMG KN KWARA 9 IJEBU-NORTH JGB OG 30 OYO-EAST YYY OY YOBE 1 Stanbic IBTC Pension Managers Limited 0021 29 URU OFFONG ORUKO UFG AK 7 DAMBOA DAM BO 9 ETSAKO-WEST AUC ED 21 ORLU RLU IM 33 ROGO RGG KN S/N LGA NAME LGA STATE 10 IJEBU-NORTH-EAST JNE OG 31 SAKI-EAST GMD OY S/N LGA NAME LGA STATE 2 Premium Pension Limited 0022 30 URUAN DUU AK 8 DIKWA DKW BO 10 IGUEBEN GUE ED 22 ORSU AWT IM 34 SHANONO SNN KN CODE CODE 11 IJEBU-ODE JBD OG 32 SAKI-WEST SHK OY CODE CODE 3 Leadway Pensure PFA Limited 0023 31 UYO UYY AK 9 GUBIO GUB BO 11 IKPOBA-OKHA DGE ED 23 ORU-EAST MMA IM 35 SUMAILA SML KN 1 ASA AFN KW 12 IKENNE KNN OG 33 SURULERE RSD OY 1 BADE GSH YB 4 Sigma Pensions Limited 0024 10 GUZAMALA GZM BO 12 OREDO BEN ED 24 ORU-WEST NGB IM 36 TAKAI TAK KN 2 BARUTEN KSB KW 13 IMEKO-AFON MEK OG 2 BOSARI DPH YB 5 Pensions Alliance Limited 0025 ANAMBRA 11 GWOZA GZA BO 13 ORHIONMWON ABD ED 25 OWERRI-MUNICIPAL WER IM 37 TARAUNI TRN KN 3 EDU LAF KW 14 IPOKIA PKA OG PLATEAU 3 DAMATURU DTR YB 6 ARM Pension Managers Limited 0026 S/N LGA NAME LGA STATE 12 HAWUL HWL BO 14 OVIA-NORTH-EAST AKA ED 26 26 OWERRI-NORTH RRT IM 38 TOFA TEA KN 4 EKITI ARP KW 15 OBAFEMI OWODE WDE OG S/N LGA NAME LGA STATE 4 FIKA FKA YB 7 Trustfund Pensions Plc 0028 CODE CODE 13 JERE JRE BO 15 OVIA-SOUTH-WEST GBZ ED 27 27 OWERRI-WEST UMG IM 39 TSANYAWA TYW KN 5 IFELODUN SHA KW 16 ODEDAH DED OG CODE CODE 5 FUNE FUN YB 8 First Guarantee Pension Limited 0029 1 AGUATA AGU AN 14 KAGA KGG BO 16 OWAN-EAST -

The Syntax of Po Tangle Numerals
Mary Chimaobi Amaechi 35 Journal of Universal Language 15-2 September 2014, 35-54 The Syntax of Po Tangle Numerals 1 Mary Chimaobi Amaechi University of Ilorin, Nigeria Abstract This paper examines the endangered numerals of Po Tangle [taŋglɛ], a minority language spoken in four local government areas in Gombe State, north eastern Nigeria. The emphasis is on the cardinal whole numbers. The study explores the structure of complex numerals which are derived from simple lexical ones using syntactic coordination and complementation. The study adopts the packing strategy framework of Hurford (1975, 1987, 2003, 2007). This framework adopted applies very widely to numeral systems that uses syntactic constructions to signal multiplication and addition. It is found out that there is no overt marker for multiplicative arithmetic operation but there are two distinct markers for additive arithmetic operation, salai and ka, while the former is used for lower complex numerals in the base of ten, the latter is found in higher complex numerals of bases hundred and thousand. Keywords: Po Tangle, numeral, packing strategy, syntax, connectives, phrase structure rules Mary Chimaobi Amaechi Department of Linguistics and Nigerian Languages, University of Ilorin P.M.B. 1515, Ilorin, Nigeria Phone: +2348066392599; Email: [email protected] Received June 12, 2014; Revised July 30, 2014; Accepted August 28, 2014. 36 The Syntax of Po Tangle Numerals 1. Introduction In everyday life, human beings use numbers to make reference to time, quantity, distance, weight, height, and so on. It is the case that the numeral systems of most of the world’s languages are more endangered than the languages themselves. -
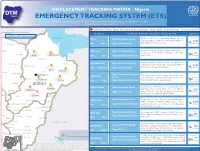
ETS) IOM OIM the DTM Emergency Tracking Tool (ETS) Is Deployed to Track and Provide Up-To-Date Information on Sudden Displacement and Return Movements
DISPLACEMENT TRACKING MATRIX - Nigeria DTM Nigeria EMERGENCY TRACKING SYSTEM (ETS) IOM OIM The DTM Emergency Tracking Tool (ETS) is deployed to track and provide up-to-date information on sudden displacement and return movements CHAD SNAPSHOT: Pulka, Bama, Monguno, Gubio, Konduga, Dikwa, Mafa, Chibok, Gwoza - February 10, 2017 NIGER Checkpoint Characteristic of movement (January 27 - February 10, 2017) Population LOCATIONS WITH IDP MOVEMENT Lake chad Estimate PULKA Type of movement: Arrivals Inow of IDPs from neighboring villages due to ongoing military activity in nearby Ngoshi village in 3,000 Gwoza LGA . Shelters are needed. persons Yunusari LGA: Gwoza Trigger: military activity Estimate Bursari BAMA Town Type of movement: Arrivals A total of 67 People returned to Nigeria from cameroon on January 29 (19 IDPs) & February 2 (48 IDPs) 67 Geidam Gubio Monguno LGA: Bama Trigger: Newly accessible areas respectively. persons Nganzai Estimate MONGUNO Town Type of movement: Arrivals Ongoing military oensive in Marte has opened up Tarmua Ngala villages previously inaccessible causing daily inux of Magumeri 8,000 LGA: Monguno Trigger: military activity IDPs towards Monguno from Marte. persons Mafa Jere Dikwa Fune Damaturu Maiduguri Type of movement: Anticipated Estimate GUBIO Town The military announced last week that people could Potiskum returns start returning to Ngetra, Kingowa, Feto, Zowo and Returns Kaga are Konduga Bama LGA: Gubio Trigger: Newly accessible areas Ardimin Wards. Returns are anticipated. anticipated Gujba Fika BORNO Estimate KONDUGA Town Type of movement: Arrivals Galtimari area of Konduga has received about 1,200 Gwoza individuals from Kawuri Village from the 27th of Damboa 1,827 Nafada Gulani LGA: Konduga Trigger: military activity January till date. -

A Wet Season Survey of Animal Trypanosomosis in Shongom Local Government Area of Gombe State. Nigeria
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Obihiro University of Agriculture and Veterinary Medicine Academic Repository A wet season survey of animal trypanosomosis in Shongom local government area of Gombe state. Nigeria 著者(英) Shamaki B.U, Obaloto O.B, Kalejaiye J.O, Lawani F.A.G, Balak G.G, Charles D journal or The journal of protozoology research publication title volume 19 number 2 page range 1-6 year 2009-12 URL http://id.nii.ac.jp/1588/00001449/ J. Protozool. Res. 19, 1-6 (2009) Copyright 2008, National Research Center for Protozoan Diseases A wet season survey of animal trypanosomosis in Shongom local government area of Gombe state. Nigeria Shamaki, B.U.1*, Obaloto, O.B.1, Kalejaiye, J.O.1, Lawani, F.A.G.2, Balak, G.G.1 and Charles D.1 1Nigerian Institute for Trypanosomiasis and Onchocerciasis Research (NITOR) Vom., 2NITOR, Kaduna, Nigeria *Corresponding author: Dr. Shamaki Bala Usman,E-mail: [email protected]. ABSTRACT Two hundred and three (203) blood samples were collected from randomly selected herd comprising; cattle, 68 (33.5%), sheep 57 (28.1%), goats 16 (7.9%), donkey, 3 (1.5%) and pigs 59 (29.1%) respectively. The blood samples were collected from animals and examined in seven villages from two districts (Filiya and Lapan) of Shongom Local Government Area. These total numbers of 203 comprises 47 (23.2%) males and 156 (76.8%) females. From the males, 15 (31.9%) were cattle, 11 (23.4%) sheep, 5 (10.6%) goats, 0 (0.0%) donkey and 16 (34.0%) boar while the females consist of 53 (34.0%) cattle, 46 (29.5%) sheep, 11 (7.1%) goats, 3 (1.9%) donkeys and 43 (27.6%) sow. -

PSWG Actors July 2016
protectionsector COMPLETED AND W O R K I N G G R O U P NIGERIA: PROTECTION ACTORS ON-GOING ACTIVITIES N I G E R I A Agencies with registered projects in OCHA Online Project Systems (OPS) JAN - JUL 2016 COOPI (Cooperazione Internazionale) DRC (Danish Refugee Council) IOM (International Organization for Migration) POPULATION POPULATION POPULATION REACHED 2,818 REACHED 13,363 REACHED 70,246 IMPLEMENTING PARTNERS IMPLEMENTING PARTNERS IMPLEMENTING PARTNERS YOBE BORNO Direct Implementation YOBE BORNO Direct Implementation YOBE BORNO Direct Implementation 2,818 10,988 46,896 MAIDUGURI MAIDUGURI DAMATURU POTISKUM FIKA BENEFICIARIES PER ACTIVITY CHIBOK GOMBE GOMBE MICHIKA GOMBE MUBI 2 Case Referrals NORTH GIRERI BENEFICIARIES PER ACTIVITY 54 Capacity Building BENEFICIARIES PER ACTIVITY Unaccompanied and ADAMAWA 947 Multiple Needs ADAMAWA ADAMAWA 2 63 Livelihood Separated Children YOLA YOLA YOLA SOUTH NORTH Unaccompanied and YOLA Protection SOUTH 19 Multiple Needs 1,871 NORTH 82 Separated Children Mainstreaming 2,375 23,350 108 Capacity Building 176 Awareness Raising / Sensitization 361 Case Referrals 271 Material Protection Assistance Psychosocial Distress Identification of 69,756 and Mental Disorder 3 6 1,727 Vulnerable Individuals 3 LOCAL GOVERNMENT LOCAL GOVERNMENT LOCAL GOVERNMENT UNIT COVERED UNIT COVERED 10,988 Dangers and Injuries UNIT COVERED NRC IRC (International Rescue Committee) NRC (Norwegian Refugee Council) Mercy Corps POPULATION POPULATION POPULATION REACHED 111,750 REACHED 224 REACHED 6,393 IMPLEMENTING PARTNERS IMPLEMENTING -

Gombe State Framework for the Implementation of Expanded Access to Family Planning Services 2013‒2018
Gombe State Framework for the Implementation of Expanded Access to Family Planning Services 2013-2018 December 2012 December 2012 Gombe State Framework for the Implementation of Expanded Access to Family Planning Services 2013-2018 December 2012 The Gombe State Framework for the Implementation of Expanded Access to Family Planning Services 2013 2018 was developed by the Gombe State Ministry of Health in July 2012. Financial Assistance for the framework was provided by the U.S. Agency for International Development (USAID) under the terms of the‒ Cooperative Agreement GPO – A-00-08-00001-00 through FHI 360’s Program Research for Strengthening Services (PROGRESS) project. The contents of this publication do not necessarily reflect the views of USAID or the U.S. government. This publication may be freely reviewed, quoted, reproduced or translated, in full or in part, provided the source is acknowledged. ©2012 Gombe State Ministry of Health, Nigeria First published in 2012 by the Gombe State Ministry of Health, Nigeria, with support from USAID– PROGRESS Citation: Gombe State Ministry of Health (SMoH). 2012. Gombe state framework for the implementation of expanded access to family planning services. Gombe (Nigeria): SMoH; 2012 Dec. TABLE OF CONTENTS LE OF CONTENTS Table of contents ................................................................................................................................... 1 Foreword ...............................................................................................................................................