Invasive Insects E Insects of Concern to Georgia
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Topic Paper Chilterns Beechwoods
. O O o . 0 O . 0 . O Shoping growth in Docorum Appendices for Topic Paper for the Chilterns Beechwoods SAC A summary/overview of available evidence BOROUGH Dacorum Local Plan (2020-2038) Emerging Strategy for Growth COUNCIL November 2020 Appendices Natural England reports 5 Chilterns Beechwoods Special Area of Conservation 6 Appendix 1: Citation for Chilterns Beechwoods Special Area of Conservation (SAC) 7 Appendix 2: Chilterns Beechwoods SAC Features Matrix 9 Appendix 3: European Site Conservation Objectives for Chilterns Beechwoods Special Area of Conservation Site Code: UK0012724 11 Appendix 4: Site Improvement Plan for Chilterns Beechwoods SAC, 2015 13 Ashridge Commons and Woods SSSI 27 Appendix 5: Ashridge Commons and Woods SSSI citation 28 Appendix 6: Condition summary from Natural England’s website for Ashridge Commons and Woods SSSI 31 Appendix 7: Condition Assessment from Natural England’s website for Ashridge Commons and Woods SSSI 33 Appendix 8: Operations likely to damage the special interest features at Ashridge Commons and Woods, SSSI, Hertfordshire/Buckinghamshire 38 Appendix 9: Views About Management: A statement of English Nature’s views about the management of Ashridge Commons and Woods Site of Special Scientific Interest (SSSI), 2003 40 Tring Woodlands SSSI 44 Appendix 10: Tring Woodlands SSSI citation 45 Appendix 11: Condition summary from Natural England’s website for Tring Woodlands SSSI 48 Appendix 12: Condition Assessment from Natural England’s website for Tring Woodlands SSSI 51 Appendix 13: Operations likely to damage the special interest features at Tring Woodlands SSSI 53 Appendix 14: Views About Management: A statement of English Nature’s views about the management of Tring Woodlands Site of Special Scientific Interest (SSSI), 2003. -

Coleoptera: Chrysomelidae: Alticinae) of the Fauna of Latvia
Acta Zoologica Lituanica, 2009, Volumen 19, Numerus 2 DOI: 10.2478/v10043-009-0011-x ISSN 1648-6919 TO THE KNOWLEDGE OF FLEA BEETLES (COLEOPTERA: CHRYSOMELIDAE: ALTICINAE) OF THE FAUNA OF LATVIA. 3. GENERA NEOCREPIDODERA HEIKERTINGER, 1911 AND CREPIDODERA CHEVROLAT, 1836 Andris BUKEJS Institute of Systematic Biology, Daugavpils University, Vienības 13, Daugavpils, LV-5401, Latvia. E-mail: [email protected] Abstract. Faunal data on four species of the genus Neocrepidodera Heikertinger, 1911 and on five spe- cies of the genus Crepidodera Chevrolat, 1836 are presented. A total of 806 specimens of these genera have been processed. The bibliographic information on these flea beetle genera in Latvia is summarised for the first time. One species, Crepidodera lamina (Bedel, 1901), is deleted from the list of Latvian Coleoptera. The annotated list of Latvian species is given, including five species of Neocrepidodera Heikertinger, 1911 and five species of Crepidodera Chevrolat, 1836. Key words: Coleoptera, Chrysomelidae, Alticinae, Neocrepidodera, Crepidodera, fauna, Latvia INTRODUCT I ON and Pūtele 1976; Rūtenberga 1992; Barševskis 1993, 1997; Bukejs and Telnov 2007. The most recent lists of This publication continues our study on flea beetles of Latvian Neocrepidodera and Crepidodera can be found the Latvian fauna (Bukejs 2008b, c). in the published catalogues of Latvian Coleoptera by There are 48 species and subspecies of the genus Neo- Telnov et al. (1997) and Telnov (2004), respectively. crepidodera Heikertinger, 1911 and 17 species of the The imagoes of Crepidodera feed on leaves of Salix genus Crepidodera Chevrolat, 1836 known in the Pa- and Populus. The larvae of Crepidodera aurata (Mar- laearctic region (Gruev & Döberl 1997). -

Native PA Species Shrubs and Trees Eligible for Stormwater Credits in Venango County
Native PA Species Shrubs and Trees Eligible for Stormwater Credits in Venango County. Compiled by: Judy Acker, French Creek Outreach Coordinator, Audubon Pennsylvania Amelanchier arborea Common Serviceberry 1 Plants marked with an BF Are available at: Beechwood Farms Nature Reserve Audubon Center for Native Plants 614 Dorseyville Road, Fox Chapel Borough Pittsburgh, PA 15238 Phone: 412-963-6100 All plants marked with an E Are available at: Ernst Conservation Seeds 9006 Mercer Pike Meadville, PA 16335 800-673-3231 OR 814-336-2404 (Sometimes plugs, potted and sometimes by seeds only) All plants marked with a PC Are available at: Pampas Creek Perennials Nursery & Greenhouse 6482 Galmish Road Cochranton, PA 16314 814-425-3080 All plants marked with a J Are available from: Johnston’s Evergreen Nursery Inc. 1000 Wales Road Erie, PA 16150 814-739-2820 All plants marked with a SY Are available from: Scotland Yards Greenhouse 12555 Fry Rd Edinboro, PA 16412 (814) 734-6700 **REMEMBER plant lists change because of sales and other factors so contact the Nurseries for availability. **MAKE SURE THE LATIN NAME IS CORRECT BEFORE YOU BUY THE PLANT! 2 Native PA Species Shrubs Eligible for Stormwater Credits in Venango County. Red Chokeberry-- Aronia arbutifolia / Photinia pyrifoliea —E, J Native habitat: swamps, bogs, moist woods. A colonial shrub species, grows from 3’ to 15’ tall, prefers full sun, low drought tolerance, blooms from April to July, white flowers, produces pear shaped red fruit that per- sists into winter. Moist rich acidic soils. Fall foliage red to red orange. A source of wildlife cover and fall food. -

Proceedings, 23Rd U.S. Department of Agriculture Interagency Research
United States Department of Proceedings Agriculture 23rd U.S. Department of Agriculture Forest Service Northern Interagency Research Forum on Research Station Invasive Species 2012 General Technical Report NRS-P-114 The findings and conclusions of each article in this publication are those of the individual author(s) and do not necessarily represent the views of the U.S. Department of Agriculture or the Forest Service. All articles were received in digital format and were edited for uniform type and style. Each author is responsible for the accuracy and content of his or her paper. The use of trade, firm, or corporation names in this publication is for the information and convenience of the reader. Such use does not constitute an official endorsement or approval by the U.S. Department of Agriculture or the Forest Service of any product or service to the exclusion of others that may be suitable. This publication reports research involving pesticides. It does not contain recommendations for their use, nor does it imply that the uses discussed here have been registered. All uses of pesticides must be registered by appropriate State and/or Federal, agencies before they can be recommended. CAUTION: Pesticides can be injurious to humans, domestic animals, desirable plants, and fi sh or other wildlife—if they are not handled or applied properly. Use all pesticides selectively and carefully. Follow recommended practices for the disposal of surplus pesticides and pesticide containers. Cover graphic by Vincent D’Amico, U.S. Forest Service, Northern Research Station. Manuscript received for publication August 2012 Published by: For additional copies: U.S. -

Effect of Trap Color on Captures of Bark- and Wood-Boring Beetles
insects Article Effect of Trap Color on Captures of Bark- and Wood-Boring Beetles (Coleoptera; Buprestidae and Scolytinae) and Associated Predators Giacomo Cavaletto 1,*, Massimo Faccoli 1, Lorenzo Marini 1 , Johannes Spaethe 2 , Gianluca Magnani 3 and Davide Rassati 1,* 1 Department of Agronomy, Food, Natural Resources, Animals and Environment (DAFNAE), University of Padova, Viale dell’Università, 16–35020 Legnaro, Italy; [email protected] (M.F.); [email protected] (L.M.) 2 Department of Behavioral Physiology & Sociobiology, Biozentrum, University of Würzburg, Am Hubland, 97074 Würzburg, Germany; [email protected] 3 Via Gianfanti 6, 47521 Cesena, Italy; [email protected] * Correspondence: [email protected] (G.C.); [email protected] (D.R.); Tel.: +39-049-8272875 (G.C.); +39-049-8272803 (D.R.) Received: 9 October 2020; Accepted: 28 October 2020; Published: 30 October 2020 Simple Summary: Several wood-associated insects are inadvertently introduced every year within wood-packaging materials used in international trade. These insects can cause impressive economic and ecological damage in the invaded environment. Thus, several countries use traps baited with pheromones and plant volatiles at ports of entry and surrounding natural areas to intercept incoming exotic species soon after their arrival and thereby reduce the likelihood of their establishment. In this study, we investigated the performance of eight trap colors in attracting jewel beetles and bark and ambrosia beetles to test if the trap colors currently used in survey programs worldwide are the most efficient for trapping these potential forest pests. In addition, we tested whether trap colors can be exploited to minimize inadvertent removal of their natural enemies. -

Trichoferus Campestris (Faldermann)
Velvet Longhorn Beetle Screening Aid Trichoferus campestris (Faldermann) Hanna R. Royals and Todd M. Gilligan Identification Technology Program (ITP) / Colorado State University, USDA-APHIS-PPQ-Science & Technology (S&T), 2301 Research Boulevard, Suite 108, Fort Collins, Colorado 80526 U.S.A. (Emails: [email protected]; [email protected]) This CAPS (Cooperative Agricultural Pest Survey) screening aid produced for and distributed by: Version 2.0 USDA-APHIS-PPQ National Identification Services (NIS) 29 Jan 2019 This and other identification resources are available at: http://caps.ceris.purdue.edu/taxonomic-services Trichoferus campestris (Faldermann), the velvet longhorn beetle, is a wood- boring beetle native to Asia (also in literature as Hesperophanes campestris). The recorded host plants for this beetle are numerous, encompassing at least 40 genera of woody plants. They preferentially attack apple (Malus) and mulberry (Morus), but have been recorded on Betula, Broussonetia, Gleditsia, Salix, Sorbus, and various other fruit and deciduous trees. The most likely pathway for these beetles into North America is imported wood dunnage and wood packaging, as this pest is able to develop in very dry wood. In 1997, a localized infestation of this species occurred in a storage site in New Brunswick, New Jersey. Two specimens were recorded in a residential area near Montreal in 2002, and adults and larvae were collected from dying logs of Norway maple Fig. 1: Adult of Trichoferus campestris (Photo (Acer platanoides) in Ontario. Since then, adults have been captured in Lindgren by Gyorgy Csoka, Hungary Forest Research funnel traps deployed in Illinois, Ohio, Minnesota, and Utah. -

American Hornbeam Is Planted in Landscapes and AMERICAN Naturalized Areas
Plant Fact Sheet American hornbeam is planted in landscapes and AMERICAN naturalized areas. It prefers deep, fertile, moist, acidic soil and grows best in partial shade, but will HORNBEAM grow in full sun. Its chief liabilities in cultivation are Carpinus caroliniana Walt. a relatively slow growth rate and difficulty in transplantation. It is not drought-tolerant. Plant Symbol = CACA18 Seeds, buds, or catkins are eaten by a number of Contributed by: USDA NRCS National Plant Data songbirds, ruffed grouse, ring-necked pheasants, Center & the Biota of North America Program bobwhite, turkey, fox, and gray squirrels. Cottontails, beaver, and white-tailed deer eat the leaves, twigs, and larger stems. American hornbeam is heavily used by beaver, because it is readily available in typical beaver habitat. Status Please consult the PLANTS Web site and your State Department of Natural Resources for this plant’s current status (e.g. threatened or endangered species, state noxious status, and wetland indicator values). Description American hornbeam is a native, large shrub or small tree with a wide-spreading, flat-topped crown, the stems are slender, dark brown, hairy; bark gray, thin, usually smooth, with smooth, longitudinal fluting (resembling a flexed muscle). Its leaves are deciduous, arranged alternately along stems, egg- shaped to elliptical in outline, ¾ to 4¾ inches long, with doubly-serrate edges. They are glabrous above, slightly to moderately pubescent beneath, especially on major veins, with or without conspicuous dark glands. During the growing season, leaves are dark green but turn yellow to orange or red in the fall. The flowers are unisexual, in catkins. -
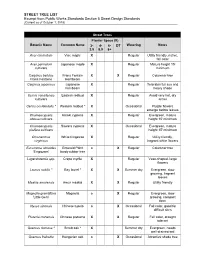
Approved Street Tree and Shrub List, from Public Works Standards
STREET TREE LIST Excerpt from Public Works Standards Section 5 Street Design Standards (Current as of October 1, 2019) Street Trees Planter Space (ft) Botanic Name Common Name 3- 4- 6- DT Watering Notes 3.9 5.9 8+ Acer circinatum Vine maple X Regular Utility friendly, native, fall color Acer palmatum Japanese maple X Regular Mature height 15' cultivars minimum Carpinus betulus Frans Fontain X X Regular Columnar tree Frans Fontaine Hornbeam Carpinus japonicus Japanese X Regular Tolerates full sun and hornbeam heavy shade Cercis canadensis Eastern redbud X Regular Avoid very hot, dry cultivars areas Cercis occidentalis * Western redbud * X Occasional Purple flowers emerge before leaves Chamaecyparis Hinoki cypress X Regular Evergreen, mature obtusa cultivars height 15' minimum Chamaecyparis Sawara cypress X Occasional Evergreen, mature pisifera cultivars height 15' minimum Chionanthus White fringetree X Regular Utility friendly, virginicus fragrant white flowers Eucommia ulmoides Emerald Point o X Regular Columnar tree 'Empozam' hardy rubber tree Lagerstroemia spp. Crepe myrtle X Regular Vase-shaped, large flowers Laurus nobilis * Bay laurel * X X Summer dry Evergreen, slow growing, fragrant leaves Maakia amurensis Amur maakia X X Regular Utility friendly Magnolia grandiflora Magnolia o X Regular Evergreen, slow 'Little Gem' growing, compact form Nyssa sinensis Chinese tupelo o X Occasional Fall color, good for difficult sites Pistacia chinensis Chinese pistache X X Regular Fall color, drought tolerant Quercus dumosa * Scrub oak * X Summer -

Insects for Weed Control: Status in North Dakota
Insects for Weed Control: Status in North Dakota E. U. Balsbaugh, Jr. Associate Professor, Department of Entomology R. D. Frye Professor, Department of Entomology C. G. Scholl Plant Protection Specialist, North Dakota State Department of Agriculture A. W. Anderson Associate Professor, Department of Entomology Two foreign species of weevils have been introduced into North Dakota for the biological control of musk thistle, Carduus nutans L. - Rhinocyllus conicus (Froelich), a seed feeding weevH, and Ceutorrhynchidius horridus (Panzer), an internal root and lower stem inhabiting species. R. conicus has survived for several generations and is showing some promise for thistle suppression in Walsh County, but releases of C. hor ridus have been unsuccessful. The pigweed nea beetle, Disonycha glabrata (Fab.), which is native in southern United States, has been introduced into experimental sugarbeet plots in the Red River Valley for testing its effects at controlling rough pigweed or redroot, Amaranthus retroflexus L. Although both larvae and adults of these beetles feed heavily on pigweed, damage to the weed occurs too late in the season for them to be effective in suppressing weed growth or seed set. An initial survey for native insects of bindweed has been conducted. Feeding by localized populations of various insects, particularly tortoise beetles, has been observed. Several foreign species of nea beetles and a stem boring beetle are anticipated for release against leafy spurge. Entomologists at North Dakota State University are successful. In 1940, the cactus-feeding moth, Cac studying insects that feed on weeds to determine if the toblastis cactorum (Berg), was transported from its insects can reduce populations of selected weeds to native Argentina to Australia where it greatly reduced levels at which the weeds no longer are economically im the numbers of prickly pear cactus, Opuntia spp. -

Insect Pest List by Host Tree and Reported Country
Insect pest list by host tree and reported country Scientific name Acalolepta cervina Hope, 1831 Teak canker grub|Eng Cerambycidae Coleoptera Hosting tree Genera Species Family Tree species common name Reported Country Tectona grandis Verbenaceae Teak-Jati Thailand Scientific name Amblypelta cocophaga Fruit spotting bug|eng Coconut Coreidae Hemiptera nutfall bug|Eng, Chinche del Hosting tree Genera Species Family Tree species common name Reported Country Agathis macrophylla Araucariaceae Kauri Solomon Islands Eucalyptus deglupta Myrtaceae Kamarere-Bagras Solomon Islands Scientific name Anoplophora glabripennis Motschulsky Asian longhorn beetle (ALB)|eng Cerambycidae Coleoptera Hosting tree Genera Species Family Tree species common name Reported Country Paraserianthes falcataria Leguminosae Sengon-Albizia-Falcata-Molucca albizia- China Moluccac sau-Jeungjing-Sengon-Batai-Mara- Falcata Populus spp. Salicaceae Poplar China Salix spp. Salicaceae Salix spp. China 05 November 2007 Page 1 of 35 Scientific name Aonidiella orientalis Newstead, Oriental scale|eng Diaspididae Homoptera 1894 Hosting tree Genera Species Family Tree species common name Reported Country Lovoa swynnertonii Meliaceae East African walnut Cameroon Azadirachta indica Meliaceae Melia indica-Neem Nigeria Scientific name Apethymus abdominalis Lepeletier, Tenthredinidae Hymenoptera 1823 Hosting tree Genera Species Family Tree species common name Reported Country Other Coniferous Other Coniferous Romania Scientific name Apriona germari Hope 1831 Long-horned beetle|eng Cerambycidae -

Carpinus Betulus - European Hornbeam (Betulaceae) ------Carpinus Betulus Is a Columnar to Teardrop-Shaped Tree
Carpinus betulus - European Hornbeam (Betulaceae) ------------------------------------------------------------------------------------------ Carpinus betulus is a columnar to teardrop-shaped tree. Twigs European Hornbeam is noted for fine and dense texture, -olive-brown and lenticeled, with ornamental winter ornamental winter bark and buds, dense summer foliage, buds that are long and partially curving around the twigs pendulous spring catkins, and unusual autumn fruits. -the twigs are similar to those of the European Beech, but the latter has winter buds that extend straight out of FEATURES the stem at a 45 degree angle. Form Trunk -medium-sized deciduous tree; the -smooth and steel gray, but having a muscled character rarely available species form maturing to its appearance at 40' tall x 30' wide, with the common cultivars more compact; USAGE species form an upright oval growth Function habit in youth, quickly becoming a -specimen or focal point tree of great symmetrical and spreading oval (low-branched teardrop architectural value; can also be an effective year-round shape) with maturity screen or tall, wide hedge when used in rows -medium growth rate Culture Texture -full sun to partial sun; prefers a well-drained soil but is -fine texture in foliage and when bare; thick density in adaptable to various soils and soil pHs; if transplanted foliage and when bare, with many ascending twigs and in autumn, use amended soil, fertilize, mulch liberally, branches forming a thick canopy even in winter and avoid winter salt spray Assets -cultivars -

Mississippi Geology 2
~ THE DEPARTMENT OF NATURAL RESOURCES- ~( miSSISSippi. ~ geology Bureau of Geology 2525 North West Street Volume 2, Number 2 • -.,.-.... Jackson, Mississippi 39216 December 1981 FOSSIL WOOD FROM THOMPSON CREEK, YAZOO COUNTY, MISSISSIPPI Will H. Blackwe ll Department of Botany and Department of Geology Miami University, Oxford, Ohio and George H. Dukes Route 2, Box 127 Brandon, Mississippi Introduction Fossil wood is a common commodity in Mississippi, even to the point that it has been designated as the "state stone." There is interest in it commercially (e.g., jewelry), as a popular collector's item among "rockhounds," and sc ientifically. Yet, available information is all too often deficient in detail (and sometimes even accuracy). This is especially true when such "hard nosed" questions as the fo ll owing are posed concerning a particular fossil wood specimen: 1) Exactly what kind of wood is it, botanically?, 2) Precisely where did it come from?, 3) How old is it, geo logically?, and 4) What is the mecha nism by which it became petrified? Certain identification, and literature providing other exact information, is typi cally lacking (or else not generally avai lable) for collec tions of petrified wood from a giveo site (outstanding exceptions are the works of E. W. Berry many years ago, e.g., Berry, 1916, 1924). Fossil wood is without question Cross section of fossil red maple (x 68) from Thompson one of Mississippi's rich natural treasures, and yet, sur- Creek. prisingly, so very little is actually known about it. This is a in particular (Fig. 1). Thompson Creek is now a rather fact which has bothered the authors of this paper for some famous local ity being as it is the site of a relatively recent time, especially since both of us grew up and received discovery of a fossi l whale (archaeocete) of Eocene Age our college educations in Mississippi, and sinc-e betA ~'lave (55 million- years B.P., approximate), which has su-bs-«? maintained an interest in southeastern fossil plant material.