Annual Report 無錫藥明康德新藥開發股份有限公司 Co., Ltd
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Chinese Pharmaceutical Startup Acquires an American Firm to “Go Global”
Paulson Papers on Investment Case Study Series A Chinese Pharmaceutical Startup Acquires an American Firm to “Go Global” June 2016 Paulson Papers on Investment Case Study Series Preface or decades, bilateral investment manufacturing—to identify tangible has flowed predominantly from the opportunities, examine constraints and FUnited States to China. But Chinese obstacles, and ultimately fashion sensible investments in the United States have investment models. expanded considerably in recent years, and this proliferation of direct investments Most of the case studies in this Investment has, in turn, sparked new debates about series look ahead. For example, our the future of US-China economic relations. agribusiness papers examine trends in the global food system and specific US and Unlike bond holdings, which can be Chinese comparative advantages. They bought or sold through a quick paper propose prospective investment models. transaction, direct investments involve people, plants, and other assets. They are But even as we look ahead, we also a vote of confidence in another country’s aim to look backward, drawing lessons economic system since they take time from past successes and failures. And both to establish and unwind. that is the purpose of the case studies, as distinct from the other papers in this The Paulson Papers on Investment aim series. Some Chinese investments in to look at the underlying economics— the United States have succeeded. They and politics—of these cross-border created or saved jobs, or have proved investments between the United States beneficial in other ways. Other Chinese and China. investments have failed: revenue sank, companies shed jobs, and, in some Many observers debate the economic, cases, businesses closed. -

Pacer CSOP FTSE China A50 ETF Schedule of Investments July 31, 2020 (Unaudited) Shares Value COMMON STOCKS - 98.0% Agriculture - 1.6% Muyuan Foodstuff Co Ltd
Page 1 of 4 Pacer CSOP FTSE China A50 ETF Schedule of Investments July 31, 2020 (Unaudited) Shares Value COMMON STOCKS - 98.0% Agriculture - 1.6% Muyuan Foodstuff Co Ltd. - Class A 9,230 $ 120,977 Wens Foodstuffs Group Co Ltd. - Class A 4,660 15,857 136,834 Auto Manufacturers - 0.7% SAIC Motor Corp Ltd. - Class A 24,600 64,077 Banks - 23.7% Agricultural Bank of China Ltd. - Class H 352,300 163,039 Bank of China Ltd. - Class H 193,900 92,512 Bank of Communications Co Ltd. - Class A 184,100 125,556 China CITIC Bank Corp Ltd. - Class H 24,700 18,261 China Construction Bank Corp. - Class H 81,500 71,464 China Everbright Bank Co Ltd. - Class H 126,400 68,456 China Merchants Bank Co Ltd. - Class A 108,200 539,489 China Minsheng Banking Corp Ltd. - Class A 254,300 201,851 Industrial & Commercial Bank of China Ltd. - Class A 198,400 140,993 Industrial Bank Co Ltd. - Class A 127,400 285,849 Ping An Bank Co Ltd. - Class A 75,000 143,348 Shanghai Pudong Development Bank Co Ltd. - Class A 132,300 196,379 2,047,197 Beverages - 17.9% Jiangsu Yanghe Brewery Joint-Stock Co Ltd. - Class A 4,000 77,398 Kweichow Moutai Co Ltd. - Class A 4,000 961,777 Wuliangye Yibin Co Ltd. - Class A 16,200 504,835 1,544,010 Building Materials - 1.6% Anhui Conch Cement Co Ltd. - Class H 15,900 139,921 Coal - 0.5% China Shenhua Energy Co Ltd. -

Schedule of Investments (Unaudited) Ishares MSCI Total International Index Fund (Percentages Shown Are Based on Net Assets) September 30, 2020
Schedule of Investments (unaudited) iShares MSCI Total International Index Fund (Percentages shown are based on Net Assets) September 30, 2020 Mutual Fund Value Total International ex U.S. Index Master Portfolio of Master Investment Portfolio $ 1,034,086,323 Total Investments — 100.4% (Cost: $929,170,670) 1,034,086,323 Liabilities in Excess of Other Assets — (0.4)% (3,643,126) Net Assets — 100.0% $ 1,030,443,197 iShares MSCI Total International Index Fund (the “Fund”) seeks to achieve its investment objective by investing all of its assets in International Tilts Master Portfolio (the “Master Portfolio”), which has the same investment objective and strategies as the Fund. As of September 30, 2020, the value of the investment and the percentage owned by the Fund of the Master Portfolio was $1,034,086,323 and 99.9%, respectively. The Fund records its investment in the Master Portfolio at fair value. The Fund’s investment in the Master Portfolio is valued pursuant to the pricing policies approved by the Board of Directors of the Master Portfolio. Fair Value Hierarchy as of Period End Various inputs are used in determining the fair value of financial instruments. These inputs to valuation techniques are categorized into a fair value hierarchy consisting of three broad levels for financial reporting purposes as follows: • Level 1 – Unadjusted price quotations in active markets/exchanges for identical assets or liabilities that the Fund has the ability to access • Level 2 – Other observable inputs (including, but not limited to, quoted prices -

China Healthcare
KURE 9/30/2019 China Healthcare: Potential Opportunities From One Of The Fastest Growing Major Global Healthcare Markets1 An Overview of the KraneShares MSCI All China Health Care Index ETF (Ticker: KURE) 1. Major healthcare markets defined as top five global markets by the World Health Organization. Data from the World Health Organization as of 12/31/2016, last updated on 1/8/2019, retrieved on 9/30/2019. [email protected] 1 Introduction to KraneShares About KraneShares Krane Funds Advisors, LLC is the investment manager for KraneShares ETFs. Our suite of China focused ETFs provides investors with solutions to capture China’s importance as an essential element of a well-designed investment portfolio. We strive to provide innovative, first to market strategies that have been developed based on our strong partnerships and our deep knowledge of investing. We help investors stay up to date on global market trends and aim to provide meaningful diversification. Krane Funds Advisors, LLC is majority owned by China International Capital Corporation (CICC). 2 Investment Strategy: KURE seeks to measure the performance of MSCI China All Shares Health Care 10/40 Index. The Index is a free float adjusted market capitalization weighted index designed to track the equity market performance of Chinese companies engaged in the health care sector. The securities in the Index include all types of publicly issued shares of Chinese issuers, which are listed in Mainland China, Hong Kong and the KURE United States. Issuers eligible for inclusion must be classified under the Global Industry Classification Standard (GICS) as engaged in the healthcare sector. -
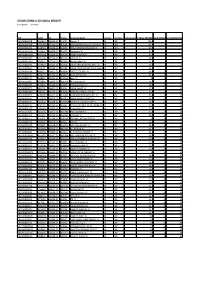
STOXX CHINA a 50 EQUAL WEIGHT Selection List
STOXX CHINA A 50 EQUAL WEIGHT Last Updated: 20210301 ISIN Sedol RIC Int.Key Company Name Country Currency Component FF Mcap (BEUR) Rank (FINAL)Rank (PREVIOUS) CNE0000018R8 6414832 600519.SS CN10RR Moutai 'A' CN CNY Y 156.4 1 1 CNE000001R84 B1SVWB6 601318.SS CN2034 PING AN INSUR GP CO. OF CN 'A' CN CNY Y 101.3 2 2 CNE000001B33 6518723 600036.SS CN2023 CHINA MERCHANTS BANK 'A' CN CNY Y 87.1 3 3 CNE000000VQ8 6109901 000858.SZ CN0EGT Wuliangye 'A' CN CNY Y 62.6 4 4 CNE100001QQ5 BDVHRJ8 000333.SZ CN40AD MIDEA GROUP 'A' CN CNY Y 57.7 5 5 CNE000001QZ7 B1P13B6 601166.SS CN01CC Ind Bank 'A' CN CNY Y 49.6 6 6 CNE0000014W7 6288457 600276.SS CN0W4Z Hengrui Medi 'A' CN CNY Y 42.3 7 7 CNE100001FR6 B759P50 601012.SS CN27DL LONGI GREEN ENERGY TECH.'A' CN CNY Y 40.4 8 9 CNE100000G29 B42G7J1 601888.SS CN0188 CHINA TOURISM GRP DUTY FREE'A'CN CNY Y 35.8 9 10 CNE0000001D4 6990257 000651.SZ CN0BHR GREE ELECT.APP. 'A' CN CNY Y 34.8 10 11 CNE1000031K4 BFXNP16 603259.SS CN84KV WUXI APPTEC 'A' CN CNY Y 31.4 11 8 CNE000001F70 6648824 600031.SS CN0QWL Sany 'A' CN CNY Y 31 12 13 CNE000000JP5 6458841 600887.SS CN00WV Yili Company 'A' CN CNY Y 30.6 13 12 CNE000001DB6 6579355 600030.SS CN0QVK CITIC Securities 'A' CN CNY Y 28.2 14 14 CNE0000000T2 6803708 000002.SZ CN06IN CHINA VANKE 'A' CN CNY Y 27.4 15 22 CNE100001RQ3 BJ0JR20 002714.SZ CN40KN MUYUAN FOODSTUFF 'A' CN CNY Y 25.8 16 27 CNE100000TP3 B64QPN3 002475.SZ CN1CMY LUXSHARE PRECISION IND. -

Wuxi Apptec Co., Ltd.* 無錫藥明康德新藥開發股份有限公司
THIS CIRCULAR IS IMPORTANT AND REQUIRES YOUR IMMEDIATE ATTENTION If you are in any doubt as to any aspect of this circular or as to the action to be taken, you should consult a stockbroker or other registered dealer in securities, a bank manager, solicitor, professional accountant or other professional adviser. If you have sold or transferred all your shares in WuXi AppTec Co., Ltd.* (無錫藥明康德新藥開發股份有限公司), you should at once hand this circular, together with the enclosed form of proxy, to the purchaser or transferee or to the bank, stockbroker or other agent through whom the sale or transfer was effected for transmission to the purchaser or transferee. Hong Kong Exchanges and Clearing Limited and The Stock Exchange of Hong Kong Limited take no responsibility for the contents of this circular, make no representation as to its accuracy or completeness and expressly disclaim any liability whatsoever for any loss howsoever arising from or in reliance upon the whole or any part of the contents of this circular. WUXI APPTEC CO., LTD.* 無錫藥明康德新藥開發股份有限公司 (A joint stock company incorporated in the People’s Republic of China with limited liability) (Stock Code: 2359) (1) WORK REPORT OF THE BOARD OF DIRECTORS FOR THE YEAR 2019; (2) WORK REPORT OF THE SUPERVISORY COMMITTEE FOR THE YEAR 2019; (3) ANNUAL REPORT FOR THE YEAR 2019; (4) FINANCIAL REPORT FOR THE YEAR 2019; (5) PROPOSED 2019 PROFIT DISTRIBUTION PLAN; (6) PROPOSED RE-ELECTION OF DIRECTORS; (7) PROPOSED ELECTION OF EXECUTIVE DIRECTOR; (8) PROPOSED RE-ELECTION OF SHAREHOLDER REPRESENTATIVE SUPERVISORS; -

SPDR® FTSE® Greater China ETF a Sub-Fund of the SPDR® Etfs Stock Code: 3073 Website
SPDR® FTSE® Greater China ETF A Sub-Fund of the SPDR® ETFs Stock Code: 3073 Website: www.spdrs.com.hk/etf/fund/fund_detail_3073_EN.html Interim Report 2021 1st October 2020 to 31st March 2021 SPDR® FTSE® Greater China ETF A Sub-Fund of the SPDR® ETFs Stock Code: 3073 Website: www.spdrs.com.hk/etf/fund/fund_detail_3073_EN.html Interim Report 2021 Contents Page Condensed Statement of Financial Position (Unaudited) 2 Condensed Statement of Comprehensive Income (Unaudited) 3 Condensed Statement of Changes in Equity (Unaudited) 4 Condensed Statement of Cash Flows (Unaudited) 5 Notes to the Unaudited Condensed Financial Statements 6 Investment Portfolio (Unaudited) 10 Statement of Movements in Portfolio Holdings (Unaudited) 41 Derivative Financial Instruments (Unaudited) 42 Performance Record (Unaudited) 42 Administration and Management 43 1 SPDR® FTSE® Greater China ETF a Sub-Fund of the SPDR® ETFs Interim Report 2021 CONDENSED STATEMENT OF FINANCIAL POSITION (UNAUDITED) As at 31st March 2021 31.03.2021 30.09.2020 Notes HK$ HK$ Assets Current assets Investments 1,385,908,557 969,438,426 Derivative financial instruments 54,331 47,229 Amounts due from brokers – 1,529,953 Dividends receivable 994,452 1,771,941 Other receivables 6(i) 122,017 347,255 Margin deposits 104,721 1,032,223 Cash at bank 6(f) 3,100,556 2,423,717 Total Assets 1,390,284,634 976,590,744 Liabilities Current liabilities Derivative financial instruments – 14 Amounts due to brokers – 1,500,560 Audit fee payable 154,612 309,225 Trustee fee payable 6(e) 382,474 270,773 Management fee payable 6(d) 684,377 481,283 Tax provision 156,265 214,282 Total Liabilities 1,377,728 2,776,137 Equity Net assets attributable to unitholders 4 1,388,906,906 973,814,607 The notes on pages 6 to 9 form part of these financial statements. -

WUXI APPTEC CO., LTD.* 無錫藥明康德新藥開發股份有限公司 (A Joint Stock Company Incorporated in the People’S Republic of China with Limited Liability) (Stock Code: 2359)
Hong Kong Exchanges and Clearing Limited and The Stock Exchange of Hong Kong Limited take no responsibility for the contents of this announcement, make no representation as to its accuracy or completeness and expressly disclaim any liability whatsoever for any loss howsoever arising from or in reliance upon the whole or any part of the contents of this announcement. WUXI APPTEC CO., LTD.* 無錫藥明康德新藥開發股份有限公司 (A joint stock company incorporated in the People’s Republic of China with limited liability) (Stock Code: 2359) ANNOUNCEMENT OF ANNUAL RESULTS FOR THE YEAR ENDED DECEMBER 31, 2019 FINANCIAL HIGHLIGHTS 2019 2018 Change RMB million RMB million Revenue 12,872.2 9,613.7 33.9% Gross Profit 5,006.1 3,776.9 32.5% Gross Profit Margin 38.9% 39.3% Net Profit Attributable to the Owners of the Company 1,854.6 2,260.5 –18.0% Margin of Net Profit Attributable to the Owners of the Company 14.4% 23.5% Adjusted Non-IFRS Net Profit Attributable to the Owners of the Company 2,407.4 1,741.6 38.2% Margin of Adjusted Non-IFRS Net Profit Attributable to the Owners of the Company 18.7% 18.1% Earnings per Share — Basic 1.14 1.59 –28.3% — Diluted 1.12 1.58 –29.1% Adjusted Non-IFRS Earnings per Share — Basic 1.48 1.23 20.3% — Diluted 1.46 1.22 19.7% – 1 – • The Board proposes the profit distribution plan for the year ended December 31, 2019 as follows: (1) a cash dividend of RMB3.37 (inclusive of tax) for every 10 Shares (representing an aggregate amount of RMB556,429,640.95 (inclusive of tax) based on the total issued Shares of the Company as of the date of this announcement), and (2) 4 new Shares for every 10 existing Shares of the Company to be issued out of reserve to all Shareholders. -

Interim Report 2019
wuxi apptec IR2019 Cover 12.9mm output.pdf 1 16/9/2019 下午4:56 無錫藥明康德新藥開發股份有限公司 無錫藥明康德新藥開發股份有限公司 WuXi AppTec Co., Ltd.* WuXi AppTec Co., Ltd.* (於中華人民共和國註冊成立的股份有限公司) (A joint stock company incorporated in the People’s Republic of China with limited liability) 股份代碼:2359 Stock Code : 2359 W uXi App C M Y T CM ec Co., Ltd. MY CY CMY K * INTERIM REPORT INTERIM REPORT INTERIM 中期報告 2019 REPORT 2019 中期報告 2019 CONTENTS CORPORATE INFORMATION 2 FINANCIAL HIGHLIGHTS 4 MANAGEMENT DISCUSSION AND ANALYSIS 5 STATUTORY DISCLOSURES 31 REPORT ON REVIEW OF CONDENSED CONSOLIDATED FINANCIAL STATEMENTS 47 CONDENSED CONSOLIDATED STATEMENT OF PROFIT OR LOSS AND OTHER COMPREHENSIVE INCOME 48 CONDENSED CONSOLIDATED STATEMENT OF FINANCIAL POSITION 50 CONDENSED CONSOLIDATED STATEMENT OF CHANGES IN EQUITY 52 CONDENSED CONSOLIDATED STATEMENT OF CASH FLOWS 54 NOTES TO THE CONDENSED CONSOLIDATED FINANCIAL STATEMENTS 56 DEFINITIONS 110 CORPORATE INFORMATION BOARD OF DIRECTORS REMUNERATION AND APPRAISAL Executive Directors COMMITTEE Dr. Ge Li (李革) Ms. Yan Liu (劉艷) (Chairperson) 婁賀統 (Chairman and Chief Executive Officer) Dr. Hetong Lou ( ) Mr. Edward Hu (胡正國) Dr. Ning Zhao (趙寧) (Co-Chief Executive Officer) Mr. Xiaozhong Liu (劉曉鐘) NOMINATION COMMITTEE 張朝暉 Mr. Zhaohui Zhang ( ) Dr. Jiangnan Cai (蔡江南) (Chairperson) 趙寧 Dr. Ning Zhao ( ) Ms. Yan Liu (劉艷) Dr. Ge Li (李革) Non-executive Directors Mr. Xiaomeng Tong (童小幪) AUDITOR Dr. Yibing Wu (吳亦兵) Deloitte Touche Tohmatsu Certified Public Accountants Independent Non-executive Directors 35/F, One Pacific Place Dr. Jiangnan Cai (蔡江南) 88 Queensway Ms. Yan Liu (劉艷) Hong Kong Mr. Dai Feng (馮岱) Dr. Hetong Lou (婁賀統) REGISTERED OFFICE IN THE PRC Mr. -

Holdings Portfolio
AMG Veritas Asia Pacific Fund - Portfolio Holdings as of August 31, 2021 Ticker Name Sector Asset Class Country Currency Par/Shares Price ($) Market Value ($) % of Fund 2330 Taiwan Semiconductor Manufacturing Co Ltd Information Technology Stock TW TWD 771,400 21.94 $16,920,843 7.99% 005930 Samsung Electronics Co Ltd Information Technology Stock KR KRW 217,650 66.02 $14,368,868 6.79% SE Sea Ltd ADR Communication Services Stock SG USD 31,636 338.32 $10,703,092 5.06% HDB HDFC Bank Ltd ADR Financials Stock IN USD 126,816 78.31 $9,930,961 4.69% 300274 Sungrow Power Supply Co Ltd Industrials Stock CN CNY 394,700 24.32 $9,599,719 4.54% 051910 LG Chem Ltd Materials Stock KR KRW 13,040 651.61 $8,496,947 4.01% 006400 Samsung SDI Co Ltd Information Technology Stock KR KRW 12,400 681.59 $8,451,682 3.99% APHS Apollo Hospitals Enterprise Ltd PNote Health Care Stock IN USD 118,000 67.97 $8,019,906 3.79% APT Afterpay Ltd Information Technology Stock AU AUD 81,597 97.27 $7,936,897 3.75% 600519 Kweichow Moutai Co Ltd, Class A Consumer Staples Stock CN CNY 31,000 241.27 $7,479,375 3.53% HUVR Hindustan Unilever Ltd PNote Consumer Staples Stock IN USD 194,500 37.24 $7,243,874 3.42% 300124 Shenzhen Inovance Technology Co Ltd Industrials Stock CN CNY 610,147 11.00 $6,712,199 3.17% TCS Tata Consultancy Services Ltd PNote Information Technology Stock IN USD 120,731 51.77 $6,249,815 2.95% 300450 Wuxi Lead Intelligent Equipment Co Ltd, Class A Information Technology Stock CN CNY 539,998 11.52 $6,223,061 2.94% 000858 Wuliangye Yibin Co Ltd, Class A Consumer Staples -

Consumer Discretionary (OUTPERFORM)
绝对保密 Consumer Discretionary (OUTPERFORM) Walter WOO (852) 9845 6974 / 3761 8776 [email protected] Jan 2020 Contents Part 1 Investment summary and Top picks Part 2 Positive on 2020E, led by faster income and wealth growth Part 3 Outperform: Appliances, Luxury, Tourism and Sportswear Part 4 Market-perform: Catering, Apparel and Textiles & Exports Part 5 Valuation table 1 Investment summary and Top picks • Industry trend : Positive on 2020E, led by faster income and wealth growth – 2019 retail sales growth slowed again, but quality picks continue to outperform (Sports, Catering and Appliances). – Positive on 2020E demand, thanks to accelerated income and wealth growth. – Home Appliance & Furniture (+ve) to recover in 2020E, derived by property completion cycle and wealth effect. – Luxury goods (+ve)’s growth should be decent in 2020E, thanks to premiumization. – Tourism (+ve)’s growth (ex-HK/Macau) accelerated in 2019 and should be fast in 2020E (includes HK/ Macau). – Sportswear (+ve) leaders shall keep on outperform and gain shares in 2020E by product and branding upgrades. • Top picks : Haier Electronics, Midea, Prada, Samsonite, Li Ning, Lifestyle – Haier Electronics (1169 HK, NR, at 13x FY20E P/E): 1) potential privatization, 2) premiumization (accelerated growth from Casarte), and 3) low base last year. – Midea (000333 CH, BUY, TP: RMB75.33, on 18x FY20E P/E): 1) excellent and diversified product mix, 2) meaningful exposure in small appliance and 3) rightly placed incentives. – Prada (1913 HK, BUY, TP: HK$ 31.39, on 37x FY20E P/E): 1) better sales growth despite drag from HK, 2) greater efforts in marketing (e.g. -

上海復星醫藥(集團)股份有限公司 Shanghai Fosun Pharmaceutical
Hong Kong Exchanges and Clearing Limited and The Stock Exchange of Hong Kong Limited take no responsibility for the contents of this announcement, make no representation as to its accuracy or completeness and expressly disclaim any liability whatsoever for any loss howsoever arising from or in reliance upon the whole or any part of the contents of this announcement. 上海復星醫藥(集團)股份有限公司 Shanghai Fosun Pharmaceutical (Group) Co., Ltd.* (a joint stock limited company incorporated in the People’s Republic of China with limited liability) (Stock Code: 02196) OVERSEAS REGULATORY ANNOUNCEMENT This announcement is made pursuant to Rule 13.10B of the Rules Governing the Listing of Securities on The Stock Exchange of Hong Kong Limited. The following sets out the “Announcement in Relation to the Approval for Clinical Trial regarding Investigational New Drug of a Subsidiary” published by Shanghai Fosun Pharmaceutical (Group) Co., Ltd.* (the “Company”) on the website of the Shanghai Stock Exchange, for your reference only. The following is a translation of the abovementioned announcement solely for the purpose of providing information. Should there be any discrepancies, the Chinese version will prevail. By order of the Board Shanghai Fosun Pharmaceutical (Group) Co., Ltd.* Wu Yifang Chairman Shanghai, the PRC 9 August 2021 As at the date of this announcement, the executive director of the Company is Mr. Wu Yifang; the non-executive directors of the Company are Mr. Chen Qiyu, Mr. Yao Fang, Mr. Xu Xiaoliang, Mr. Gong Ping, Mr. Pan Donghui and Mr. Zhang Houlin; and the independent non-executive directors of the Company are Ms. Li Ling, Mr.