Hormonal Responses to Non-Nutritive Sweeteners in Water and Diet Soda Allison C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Acesulfame Potassium
ACESULFAME POTASSIUM Prepared at the 57th JECFA (2001) and published in FNP 52 Add 9 (2001), superseding specifications prepared at the 46th JECFA (1996) and published in FNP 52 Add 4 (1996). An ADI of 0-15 mg/kg body weight was established at the 37th JECFA (1990). SYNONYMS Acesulfame K; INS No. 950 DEFINITION Chemical names Potassium salt of 6-methyl-1,2,3-oxathiazine-4(3H)-one-2,2-dioxide; potassium salt of 3,4-dihydro-6-methyl-1,2,3-oxathiazine-4-one-2,2-dioxide C.A.S. number 55589-62-3 Chemical formula C4H4KNO4S Structural formula Formula weight 201.24 Assay Not less than 99.0% and not more than 101.0% on the dried basis DESCRIPTION Odourless, white crystalline powder FUNCTIONAL USES Sweetener, flavour enhancer CHARACTERISTICS IDENTIFICATION Solubility (Vol. 4) Freely soluble in water, very slightly soluble in ethanol Spectrophotometry Dissolve 10 mg of the sample in 1,000 ml of water. The solution shows an absorbance maximum at 227±2 nm Test for potassium Passes test (Vol.4) Test the residue obtained by igniting 2 g of the sample Precipitation test Add a few drops of a 10% solution of sodium cobaltinitrite to a solution of 0.2 g of the sample in 2 ml of acetic acid TS and 2 ml of water. A yellow precipitate is produced. PURITY Loss on drying (Vol. 4) Not more than 1.0% (105o, 2 h) pH (Vol. 4) 5.5 - 7.5 (1% soln) Organic impurities Passes test for 20 mg/kg of UV active components See description under TESTS Fluoride (Vol. -

Royal Crown Bottling Company Of
ROYAL CROWN BOTTLING COMPANY OF WINCHESTER, INCORPORATED 10/17/12 TELEPHONE NUMBER (540) 667-1821 FAX NUMBER (540) 667-8040 Wholesale Price List ROYAL CROWN BOTTLING COMPANY OF CHARLES TOWN, INCORPORATED TELEPHONE NUMBER(304) 725-8100 FAX NUMBER (304) 725-9413 PRODUCT/PACKAGE/UPC CODES WHOLESALE PRICE SUGGESTED RETAILS Mt Dew Energy Drinks (plastic) AMP Focus Mixed Berry 16 oz can (12 loose) $19.20 $1.99 012000126338 Item: 133540 VA , MD & WV 20% Margin of Profit AMP Boost Grape 16 oz can (12 loose) $19.20 $1.99 012000382505 Item: 133541 VA, MD & WV 20% Margin of Profit AMP Boost Cherry 16 oz can (12 loose) $1.99 19.20 012000126352 Item: 133542 VA , MD & WV 20% Margin of Profit AMP Boost Original 16 oz 12 pk $1.99 $19.20 012000016431 Item: 133505 20% Margin of Profit VA,MD&WV AMP Boost Original16 oz. 6-4pks $7.89 $38.00 012000017568 Item:133510 VA,MD&WV 20% Margin of Profit PRODUCT/PACKAGE/UPC CODES WHOLESALE PRICE SUGGESTED RETAILS DEER PARK NATURAL SPRING WATER 20 oz Non-Returnable (24-loose) $19.80 $1.19 each 082657077215 Item: 129950 VA, MD & WV 20% Margin of Profit PRODUCT/PACKAGE/UPC CODES WHOLESALE PRICE SUGGESTED RETAILS VINTAGE WATER 1 Liter Non-Returnable (12 per case) $11.00 $1.19 072521051021 Item: 129710 VA, MD & WV 20% Margin of Profit 20 oz Non-Returnable (24 loose) $12.00 $.69 072521051014 Item 129700 VA, MD & WV 20% Margin of Profit PRODUCT/PACKAGE/UPC CODES WHOLESALE PRICE SUGGESTED RETAILS 10 oz Non-Returnable Glass Bottles $10.25 6/$3.19 (4-6 pack) 20% Margin of Profit VA , MD & WV 078000001686 Item: 103100 Canada -

Hormonal Responses to Non-Nutritive Sweeteners in Water and Diet Soda. Allison C
Himmelfarb Health Sciences Library, The George Washington University Health Sciences Research Commons Exercise and Nutrition Sciences Faculty Exercise and Nutrition Sciences Publications 1-1-2016 Hormonal responses to non-nutritive sweeteners in water and diet soda. Allison C. Sylvetsky George Washington University Rebecca J Brown Jenny E Blau Mary Walter Kristina I Rother Follow this and additional works at: http://hsrc.himmelfarb.gwu.edu/sphhs_exer_facpubs Part of the Dietetics and Clinical Nutrition Commons, Exercise Science Commons, and the Nutrition Commons APA Citation Sylvetsky, A. C., Brown, R., Blau, J., Walter, M., & Rother, K. (2016). Hormonal responses to non-nutritive sweeteners in water and diet soda.. Nutrition & Metabolism [electronic resource], 13 (). http://dx.doi.org/10.1186/s12986-016-0129-3 This Journal Article is brought to you for free and open access by the Exercise and Nutrition Sciences at Health Sciences Research Commons. It has been accepted for inclusion in Exercise and Nutrition Sciences Faculty Publications by an authorized administrator of Health Sciences Research Commons. For more information, please contact [email protected]. Sylvetsky et al. Nutrition & Metabolism (2016) 13:71 DOI 10.1186/s12986-016-0129-3 RESEARCH Open Access Hormonal responses to non-nutritive sweeteners in water and diet soda Allison C. Sylvetsky1,2,3, Rebecca J. Brown1, Jenny E. Blau1, Mary Walter4 and Kristina I. Rother1* Abstract Background: Non-nutritive sweeteners (NNS), especially in form of diet soda, have been linked to metabolic derangements (e.g. obesity and diabetes) in epidemiologic studies. We aimed to test acute metabolic effects of NNS in isolation (water or seltzer) and in diet sodas. -

Simultaneous Determination of Acesulfame-K, Aspartame and Stevioside in Sweetener Blends by Ultraviolet Spectroscopy with Variable Selection by Sipls Algorithm
Macedonian Journal of Chemistry and Chemical Engineering, Vol. 31, No. 1, pp. 17–28 (2012) MJCCA9 – 588 ISSN 1857-5552 UDC: 664.162.8:543.422.3-76 Received: June 5, 2011 Accepted: January 25, 2012 Original scientific paper SIMULTANEOUS DETERMINATION OF ACESULFAME-K, ASPARTAME AND STEVIOSIDE IN SWEETENER BLENDS BY ULTRAVIOLET SPECTROSCOPY WITH VARIABLE SELECTION BY SIPLS ALGORITHM Yang-Chun He, Sheng Fang*, Xue-Jiao Xu College of Food Science and Biotechnology Engineering, Zhejiang Gongshang University, Hangzhou 310035, China [email protected] A chemometric-assisted UV absorption spectroscopic method is proposed for the simultaneous de- termination of acesulfame-K, aspartame and stevioside in raw powder mixtures of commercial sweeteners. The synergy interval partial least squares (siPLS) algorithm was applied to select the optimum spectral range and their combinations. The utilization of spectral region selection aims to construct better partial least squares (PLS) model than that established from the full-spectrum range. The results show that the siPLS algorithm can find out an optimized combination of spectral regions, yielding lower relative stand- ard error of prediction (RSEP) and root mean square error of prediction (RMSEP), as well as simplifying the model. The RMSEP and RSEP obtained after selection of intervals by siPLS were 0.1330 µg·ml–1 and 1.50 % for acesulfame-K, 0.2540 µg·ml–1 and 1.64 % for aspartame, 1.4041 µg·ml–1 and 2.03 % for ste- vioside respectively. The recovery values range from 98.12 % to 101.88 % for acesulfame-K, 98.63 % to 102.96% for aspartame, and 96.38 % to 104.04 % for stevioside respectively. -

All Products Are Pareve Unless Indicated D=Dairy Or M=Meat
New to All products are pareve unless indicated D=Dairy or M=Meat. Due to limited space, this list contains only products manufactured by companies and/or plants certified within the last three months. Brands listed directly beneath one another indicate that the product list immediately below is identical for all brands. PR ODUCTS ARE CERTIF I E D ONLY WH EN BEARING TH E SYMBOL Compiled by Zeh a va Ful d a 4c Seltzer Citrus Mist Green Tea Cappuccino French Vanilla Iced Tonic Water Golden Cola Champagne Green Tea W/ginseng & Plum Juice Tea Mix ........................................D Tropical Punch Wild Cherry Seltzer Green Tea W/honey & Ginseng Cappuccino Mix-coffee Flavor..........D Vanilla Cream Soda Green Tea With Ginseng & Asia Plum Cappuccino Mix-mocha Flavor........D Wildberry Seltzer American Dry Green Tea With Ginseng And Honey Iced Tea Mix-decaffeinated Yellow Lightning Club Soda Green Tea With Honey (64oz) Iced Tea Mix-lemon Flavor Green Tea With Honey And Ginseng Anderson Erickson Iced Tea Mix-peach Flavor Adirondack Clear ‘n’ Natural Honey Lemon Premium Tea Blue Raspberry Fruit Bowl................D Iced Tea Mix-raspberry Flavor Blackberry Soda Kahlua Iced Coffee ..........................D Lite Egg Nog....................................D Iced Tea Mix-sugar Free Cherry Soda Latte Supreme..................................D Lemonade Flavor Drink Mix Cranberry Soda Lemon Iced Tea Diet Cranberry Soda Anytime Drink Crystals Lemon Tea A & W Diet Loganberry Soda Lemonade W/10% Real Lemon Juice Cream Soda Diet Raspberry Lime Soda -

Review on Artificial Sweeteners Used in Formulation of Sugar Free Syrups
International Journal of Advances in Pharmaceutics ISSN: 2320–4923; DOI: 10.7439/ijap Volume 4 Issue 2 [2015] Journal home page: http://ssjournals.com/index.php/ijap Review Article Review on artificial sweeteners used in formulation of sugar free syrups Afaque Raza Mehboob Ansari*, Saddamhusen Jahangir Mulla and Gosavi Jairam Pramod Department of Quality Assurance, D.S.T.S. Mandal’s College of Pharmacy, Jule Solapur-1, Bijapur Road, Solapur- 413004, Maharashtra, India. *Correspondence Info: Abstract Prof. Afaque Raza Mehboob Ansari Sweetening agents are employed in liquid formulations designed for oral Department of Quality Assurance, administration specifically to increase the palatability of the therapeutic agent. The D.S.T.S. Mandal’s College of main sweetening agents employed in oral preparations are sucrose, liquid glucose, Pharmacy, Jule Solapur-1, Bijapur glycerol, Sorbitol, saccharin sodium and aspartame. The use of artificial Road, Solapur- 413004, Maharashtra, sweetening agents in formulations is increasing and, in many formulations, India saccharin sodium is used either as the sole sweetening agent or in combination Email: [email protected] with sugars or Sorbitol to reduce the sugar concentration in the formulation. The Keywords: use of sugars in oral formulations for children and patients with diabetes mellitus is to be avoided. The present review discusses about the Artificial sweetening agents Sugar free syrup, which are generally used while the preparation of Sugar-free Syrup. Artificial sweeteners, Diabetes mellitus, Sucralose, and Aspartame. 1. Introduction Syrups are highly concentrated, aqueous solutions of sugar or a sugar substitute that traditionally contain a flavoring agent, e.g. cherry syrup, cocoa syrup, orange syrup, raspberry syrup. -
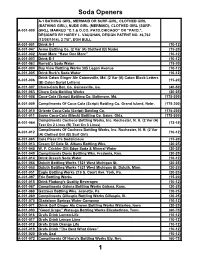
Soda Handbook
Soda Openers A-1 BATHING GIRL, MERMAID OR SURF-GIRL, CLOTHED GIRL (BATHING GIRL), NUDE GIRL (MERMAID), CLOTHED GIRL (SURF- A-001-000 GIRL), MARKED “C.T.& O.CO. PATD.CHICAGO” OR “PATD.”, DESIGNED BY HARRY L. VAUGHAN, DESIGN PATENT NO. 46,762 (12/08/1914), 2 7/8”, DON BULL A-001-001 Drink A-1 (10-12) A-001-047 Acme Bottling Co. (2 Var (A) Clothed (B) Nude) (15-20) A-001-002 Avon More “Have One More” (10-12) A-001-003 Drink B-1 (10-12) A-001-062 Barrett's Soda Water (15-20) A-001-004 Bay View Bottling Works 305 Logan Avenue (10-12) A-001-005 Drink Burk's Soda Water (10-12) Drink Caton Ginger Ale Catonsville, Md. (2 Var (A) Caton Block Letters A-001-006 (15-20) (B) Caton Script Letters) A-001-007 Chero-Cola Bot. Co. Gainesville, Ga. (40-50) A-001-063 Chero Cola Bottling Works (20-25) A-001-008 Coca-Cola (Script) Bottling Co. Baltimore, Md. (175-200) A-001-009 Compliments Of Coca-Cola (Script) Bottling Co. Grand Island, Nebr. (175-200) A-001-010 Oriente Coca-Cola (Script) Bottling Co. (175-200) A-001-011 Sayre Coca-Cola (Block) Bottling Co. Sayre, Okla. (175-200) Compliments Cocheco Bottling Works, Inc. Rochester, N. H. (2 Var (A) A-001-064 (12-15) Text On 2 Lines (B) Text On 3 Lines) Compliments Of Cocheco Bottling Works, Inc. Rochester, N. H. (2 Var A-001-012 (10-12) (A) Clothed Girl (B) Surf Girl) A-001-065 Cola Pleez It's Sodalicious (15-20) A-001-013 Cream Of Cola St. -

Larosa's Ingredient and Allergen Listing
LaRosa's Ingredient and Allergen Listing - 4.26.21 EGG FISH MILK PEANUT SHELLFISH SOY* TREE NUTS WHEAT PIZZA INGREDIENTS BUDDY DELUXE YOUR CHOICE OF CRUST + PIZZA SAUCE, PROVOLONE, PEPPERONI, SAUSAGE, SPICY SAUSAGE, BANANA PEPPERS, CAPOCOLLA HAM CHICKEN BACON RANCH YOUR CHOICE OF CRUST + RANCH DRESSING, PROVOLONE, CHICKEN BREAST STRIPS, RED ONION, BACON, TOMATOES DOUBLE PEPPERONI YOUR CHOICE OF CRUST + PIZZA SAUCE, PROVOLONE, PEPPERONI FOCACCIA FLORENTINE YOUR CHOICE OF CRUST + FOCACCIA SAUCE, 4 CHEESE BLEND, MUSHROOMS, SPINACH, TOMATOES, GREEN OLIVES, ROMANO HERB MIX FOCACCIA ROMA YOUR CHOICE OF CRUST + FOCACCIA SAUCE, 4 CHEESE BLEND, PEPPERONI, SAUSAGE, GROUND BEEF, CAPOCOLLA HAM, ROMANO HERB MIX HAWAIIAN YOUR CHOICE OF CRUST + PIZZA SAUCE, PROVOLONE, PINEAPPLE, CAPOCOLLA HAM, BACON, BANANA PEPPERS MEAT DELUXE YOUR CHOICE OF CRUST + PIZZA SAUCE, PROVOLONE, PEPPERONI, SAUSAGE, GROUND BEEF, CAPOCOLLA HAM, BACON ORIGINAL DELUXE YOUR CHOICE OF CRUST + PIZZA SAUCE, PROVOLONE, PEPPERONI, SAUSAGE, GREEN PEPPERS, RED ONION, GROUND BEEF VEGGIE DELUXE YOUR CHOICE OF CRUST + PIZZA SAUCE, PROVOLONE, MUSHROOMS, GREEN OLIVES, RED ONIONS, SPINACH, ROMA TOMATOES ZESTY BBQ CHICKEN YOUR CHOICE OF CRUST + BBQ SAUCE, PROVOLONE, CHICKEN BREAST STRIPS, JALAPENOS, RED ONION, BACON CREATE YOUR OWN PIZZA YOUR CHOICE OF CRUST + SAUCE + CHEESE + TOPPINGS ---> check allergens individually on the dough, sauce, cheese and toppings you choose WATER, MILK, PARMESAN CHEESE, HEAVY WHIPPING CREAM, ASIAGO CHEESE, WHEY PROTEIN CONCENTRATE, BUTTER, MODIFIED FOOD STARCH, SOY OIL, ROMANO CHEESE, SALT, ALFREDO SAUCE 0 0 X 0 0 0 0 0 XANTHAN GUM, BLACK PEPPER, GROUND NUTMEG, TURMERIC OLEORESIN (FOR COLOR) ANCHOVIES ANCHOVY FILLETS, OLIVE OIL, AND SALT 0 X 0 0 0 0 0 0 CURED WITH: WATER, SALT, SODIUM NITRITE. -

CAFFEINE? Department of Nutritional Services Brand Names Listed Are Not Intended to Be © 1986, Kaiser Foundation Hospitals, Endorsements of These Products
CAFFEINE CONTENT OF BEVERAGES, FOODS AND DRUGS Coffee Orange Soda 0 Drip, regular 106-164 mg/5 oz. Grape Soda 0 Percolated, regular 93-134 mg/5oz. Instant, regular 47-68 mg/5 oz. Non-prescription Drugs Decaffeinated 2-5 mg/5 oz. Stimulants (standard dose) Caffedrine capsules 200 mg Tea NoDoz Tablets 200 mg WHAT DO YOU 1 minute brew 21-33 mg/5oz. Vivarin Tablets 200 mg 3 minute brew 35-46 mg/5 oz. KNOW ABOUT 5 minute brew 39-50 mg/5 oz. Pain relievers (standard dose) Canned iced tea 22-36 mg/12 oz. Anacin Analgesic, Anacin Mix Strength, Anacin-3 64 mg Cocoa and Chocolate Cope 32 mg/tablet Cocoa Beverage Bufferin 0 (water mix) 2-8 mg/6 oz. Excedrin 130 mg Milk chocolate 6 mg/1 oz. Midol 64 mg Baking chocolate 35 mg/1 oz. Plain Aspirin, any brand 0 Sweet (dark) chocolate 20 mg/1 oz. Tylenol 0 Ovaltine 0 Vanquish 66 mg Postum 0 Diuretics (standard dose) Sodas mg/12 oz. can Aqua-Ban 200 mg Mr. Pibb, Diet 57 Fluidex 0 Mountain Dew 54 Permathene Water Off 200 mg Coca Cola, Diet Coke, Tab 45 Pre-Mens Forte 100 mg Shasta Cola, Regular and Diet 44 Mr. Pibb 41 Cold Remedies (standard dose) Dr. Pepper, Regular and Diet 40 Actifed 0 Pepsi Cola 38 Contac 0 Pepsi Light, Diet Pepsi 36 Comtrex 0 Diet Rite Cola 36 Coryban-D 30 mg Dristan 30 mg Royal Crown Cola 36 Royal Crown Cola, Diet 33 Neo-Synephrine Compounds 15 mg Cragmont Cola Trace Sudafed 0 7-up, Regular and Diet 0 Tr iaminicin 30 mg Sprite, Regular and Diet 0 Prescription Drugs Fanta 0 Cafergot Fresca 0 (migraine headaches) 100 mg/tablet Root Beer 0 Darvon Compound Club Soda 0 (pain reliever) 32 mg/tablet Ginger Ale 0 Fiorinal (headaches) 40 mg/tablet Tonic Water 0 Migrol (headaches) 50 mg/tablet CAFFEINE? Department of Nutritional Services Brand names listed are not intended to be © 1986, Kaiser Foundation Hospitals, endorsements of these products. -

Drinks That Eat Your Teeth
Drinks That Eat Your Teeth Drink Acid (pH) Sugar (tsp in 12oz) Caffeine (grams) Calories (in 12oz) Battery Acid 1.00 0 0 0 Stomach Acid 2.00 0 0 0 Lime Juice 2.00 0 0 1 Lemon Juice 2.20 0 0 12 Cranberry Juice 2.30 11 0 205 Gatorade-Clear 2.40 5.5 0 75 Sunny Delight 2.40 6.3 0 120 Vinegar 2.40 0 0 3 Pepsi 2.49 9.8 37 150 Lemonade (Country Time) 2.50 5.4 0 90 RC Cola 2.50 0 0 160 SoBo Tropical Sugarfree 2.50 0 0 0 Coke-Cherry 2.52 8.9 34 150 Coke-Classic 2.53 9.3 34 140 Capri Sun 2.60 5.5 0 200 SoBe Strawberry-Grape 2.60 6.5 0 60 Fruit Punch (Hi-C Blast) 2.70 5.5 0 150 Lemonade (Hi-C) 2.70 5.5 0 210 Orange Crush 2.70 10.5 0 240 Tang 2.70 5.1 0 180 Powerade 2.75 4 0 115 Coke-diet,Cherry 2.80 0 34 0 Grape Juice White (Welch's) 2.80 7.8 0 240 Mellow Yellow 2.80 10.1 51 177 Mr. Pibb 2.80 0 40 150 Orange Soda (Minute Maid) 2.80 11.2 0 180 Fruit Punch (Hawaiian) 2.82 10.2 0 120 Squirt 2.85 9.5 0 0 Tea-iced (Lipton Brisk) 2.87 7 9 50 7 up-Upside Down 2.90 6.3 0 200 Cranberry Juice-White 2.90 5.5 0 175 Dr Pepper 2.92 9.5 40 160 Gatorade 2.95 5.5 0 75 Tea-iced (Lemon sweetened Nestea) 2.97 9 16.6 90 Ginger Ale (Canada Dry) 3.00 8.25 0 120 Grape White (Diet Rite) 3.00 0 0 0 Grapefruit Juice 3.00 8.75 0 150 Kool-Aid Jammers-Cherry 3.00 5.1 0 160 Sierra Mist 3.00 5.5 0 140 Surge 3.02 10 51 170 Tea-Green (Nestea) 3.04 5 11-26 120 Pepsi One 3.05 0 36 1.5 Mountain Dew-diet, Code Red 3.10 0 0 0 Pepsi-Wild Cherry 3.10 5.7 0 240 V8 Splash-Berry Blend 3.10 5.5 0 105 Vinegar, cider 3.10 0 0 0 Fresca 3.20 0 0 0 Orange Strawberry Banana (Dole) 3.20 6.3 0 180 Propel 3.20 0.4 0 0 Tea-iced (Snapple) 3.20 7.6 31.5 120 Tea-iced (Diet Snapple ) 3.20 0 0 0 Jim Muenzenberger, D.D.S. -

Gluten-Free Food Labels
GLUTEN-FREE DIET: FOOD LABELS Identifying Gluten in Packaged Foods The Food Allergen Labeling and Consumer Protection Act states ‘wheat’ must be listed on the food label when wheat is an ingredient in the item. This is not true for oats, barley and rye; food manufacturers do not have to declare oats, barley or rye in the allergen statement. If you are unsure about a product’s ingredients avoid it. Use these tips to help you make gluten-free food choices: 1. Read the allergen statement. If the product contains wheat, look for another option. 2. Read the ingredient list. Please refer the lists below for ‘gluten-free’ and ‘gluten-containing’ ingredients to decide if the food is gluten free or not. 3. Look for a statement regarding the facility in which the food was processed. If the food was processed in a factory that also processes wheat, then look for another option. Please note that it is not required to include a statement regarding the facility in which the food was produced on the label. GLUTEN FREE ingredients * An asterisk denotes controversial and confusing ingredients. Details on these follow in “Controversial and Confusing Ingredients”. Acacia gum Carbooxymethlcellulose Malic acid Smoke flavoring* Acesulfame-potassium Carob bean Maltitol Sodium benzoate, Acetic acid Carrageenan Maltitol syrup metabisulphite, nitrate, Adipic acid Cellulose gum Maltol nitrite, sulphite Agar Citric acid Maltose Sorbate Agave Corn syrup Mannitol Sorbic acid Align Corn, corn bran, corn Methylycellulose Sorbitol Amaranth meal -
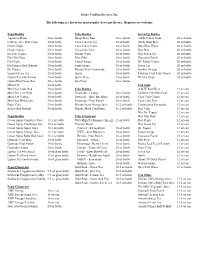
Vending-Soda-Pop-Cans.Pdf
Scioto Vending Services, Inc. The following is a list of our most popular beverage flavors. Requests are welcome. Pepsi Bottles Coke Bottles Seven Up Bottles Aquafina Water 20 oz bottle Barq's Root Beer 20 oz bottle A&W Crème Soda 20 oz bottle Caffeine-Free Diet Pepsi 20 oz bottle Coca-Cola Cherry 20 oz bottle A&W Root Beer 20 oz bottle Crush Grape 20 oz bottle Coca-Cola Classic 20 oz bottle Deja Blue Water 20 oz bottle Crush Orange 20 oz bottle Coca-Cola Zero 20 oz bottle Diet Rite 20 oz bottle Diet Dr. Pepper 20 oz bottle Dasani Water 20 oz bottle Diet Seven Up 20 oz bottle Diet Mtn Dew 20 oz bottle Diet Coke 20 oz bottle Hawaiian Punch 20 oz bottle Diet Pepsi 20 oz bottle Fanta Orange 20 oz bottle RC Royal Crown 20 oz bottle Diet Sierra Mist Natural 20 oz bottle Fanta Grape 20 oz bottle Seven Up 20 oz bottle Dr. Pepper 20 oz bottle Minute Maid Lemonade 20 oz bottle Sunkist Orange 20 oz bottle Lipton Green Tea 20 oz bottle Sprite 20 oz bottle Tahitian Treat Fruit Punch 20 oz bottle Lipton Tea with Lemon 20 oz bottle Sprite Zero 20 oz bottle Welch's Grape 20 oz bottle Lipton Diet Green Tea 20 oz bottle Sun Drop 20 oz bottle Mtn Dew 20 oz bottle Can Soda Mtn Dew Code Red 20 oz bottle Coke Bottles A & W Root Beer 12 oz can Mtn Dew Live Wire 20 oz bottle Powerade - Orange 20 oz bottle Caffeine Free Diet Coke 12 oz can Mtn Dew Voltage 20 oz bottle Powerade - Blue Mt.