Physical Controls of Variability in North Atlantic Phytoplankton Communities
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Surface Circulation2016
OCN 201 Surface Circulation Excess heat in equatorial regions requires redistribution toward the poles 1 In the Northern hemisphere, Coriolis force deflects movement to the right In the Southern hemisphere, Coriolis force deflects movement to the left Combination of atmospheric cells and Coriolis force yield the wind belts Wind belts drive ocean circulation 2 Surface circulation is one of the main transporters of “excess” heat from the tropics to northern latitudes Gulf Stream http://earthobservatory.nasa.gov/Newsroom/NewImages/Images/gulf_stream_modis_lrg.gif 3 How fast ( in miles per hour) do you think western boundary currents like the Gulf Stream are? A 1 B 2 C 4 D 8 E More! 4 mph = C Path of ocean currents affects agriculture and habitability of regions ~62 ˚N Mean Jan Faeroe temp 40 ˚F Islands ~61˚N Mean Jan Anchorage temp 13˚F Alaska 4 Average surface water temperature (N hemisphere winter) Surface currents are driven by winds, not thermohaline processes 5 Surface currents are shallow, in the upper few hundred metres of the ocean Clockwise gyres in North Atlantic and North Pacific Anti-clockwise gyres in South Atlantic and South Pacific How long do you think it takes for a trip around the North Pacific gyre? A 6 months B 1 year C 10 years D 20 years E 50 years D= ~ 20 years 6 Maximum in surface water salinity shows the gyres excess evaporation over precipitation results in higher surface water salinity Gyres are underneath, and driven by, the bands of Trade Winds and Westerlies 7 Which wind belt is Hawaii in? A Westerlies B Trade -

6 Ocean Currents 5 Gyres Curriculum
Lesson Six: Surface Ocean Currents Which factors in the earth system create gyres? Why does pollution collect in the gyres? Objective: Develop a model that shows how patterns in atmospheric and ocean currents create gyres, and explain why plastic pollution collects in them. Introduction: Gyres are circular, wind-driven ocean currents. Look at this map of the five main subtropical gyres, where the colors illustrate enormous areas of floating waste. These are places in the ocean that accumulate floating debris—in particular, plastic pollution. How do you think ocean gyres form? What patterns do you notice in terms of where the gyres are located in the ocean? The Earth is a system: a group of parts (or components) that all work together. The components of a system have different structures and functions, but if you take a component away, the system is affected. The system of the Earth is made up of four main subsystems: hydrosphere (water), atmosphere (air), geosphere (land), and biosphere (organisms). The ocean is part of our planet’s hydrosphere, but it is also its own system. Which components of the Earth system do you think create gyres, and why would pollution collect in them? Activity 1 Gyre Model: How do you think patterns in ocean currents create gyres and cause pollution to collect in the gyres? Use labels and arrows to answer this question. Keep your diagram very basic; we will explore this question more in depth as we go through each part of the lesson and you will be able to revise it. www.5gyres.org 2 Activity 2 How Do Gyres Form? A gyre is a circulating system of ocean boundary currents powered by the uneven heating of air masses and the shape of the Earth’s coastlines. -

Irish Ocean Climate and Ecosystem Status Report Summary 2009
IRISH OCEAN CLIMATE AND ECOSYSTEM STATUS REPORT SUMMARY 2009 The sea is critically important in moderating Ireland’s weather, since the majority of weather systems that affect us day to day come from the Atlantic Ocean. However, there has been very little research to date on the affects of climate change on the sea, which will inevitably impact on the various sectors that make up Ireland’s maritime economy. A fi rst step in any study of the effect of climate change on our oceans is to study the current status of Irish waters against which any future change can be measured. This can be done by examining existing data sets on oceanography, plankton and productivity, together with information on marine fi sheries and migratory species such as salmon, trout and eels. The aim of this report card is to outline the available scientifi c data on the atmosphere, oceanography, ocean chemistry, phytoplankton, zooplankton, commercial fi sheries, seabirds and migratory fi sh. Copies of the full report, Irish Ocean Climate & Ecosystem Status Report 2009, are available from Marine Institute, Rinville, Oranmore, Co. Galway, Ireland. Alternatively you can download a pdf version from www.marine.ie The Atmosphere Ireland’s climate is by no means stable in time. It is affected by a number of cyclic patterns with timescales varying in length from a year or two, to thousands of years. Some of these variations “fl ip-fl op” or oscillate between two geographical locations on a regular basis and are referred to as Atmospheric Teleconnection Patterns (ATPs). The most important of these are: 1. -

Lecture 4: OCEANS (Outline)
LectureLecture 44 :: OCEANSOCEANS (Outline)(Outline) Basic Structures and Dynamics Ekman transport Geostrophic currents Surface Ocean Circulation Subtropicl gyre Boundary current Deep Ocean Circulation Thermohaline conveyor belt ESS200A Prof. Jin -Yi Yu BasicBasic OceanOcean StructuresStructures Warm up by sunlight! Upper Ocean (~100 m) Shallow, warm upper layer where light is abundant and where most marine life can be found. Deep Ocean Cold, dark, deep ocean where plenty supplies of nutrients and carbon exist. ESS200A No sunlight! Prof. Jin -Yi Yu BasicBasic OceanOcean CurrentCurrent SystemsSystems Upper Ocean surface circulation Deep Ocean deep ocean circulation ESS200A (from “Is The Temperature Rising?”) Prof. Jin -Yi Yu TheThe StateState ofof OceansOceans Temperature warm on the upper ocean, cold in the deeper ocean. Salinity variations determined by evaporation, precipitation, sea-ice formation and melt, and river runoff. Density small in the upper ocean, large in the deeper ocean. ESS200A Prof. Jin -Yi Yu PotentialPotential TemperatureTemperature Potential temperature is very close to temperature in the ocean. The average temperature of the world ocean is about 3.6°C. ESS200A (from Global Physical Climatology ) Prof. Jin -Yi Yu SalinitySalinity E < P Sea-ice formation and melting E > P Salinity is the mass of dissolved salts in a kilogram of seawater. Unit: ‰ (part per thousand; per mil). The average salinity of the world ocean is 34.7‰. Four major factors that affect salinity: evaporation, precipitation, inflow of river water, and sea-ice formation and melting. (from Global Physical Climatology ) ESS200A Prof. Jin -Yi Yu Low density due to absorption of solar energy near the surface. DensityDensity Seawater is almost incompressible, so the density of seawater is always very close to 1000 kg/m 3. -

Ocean-Gyre-4.Pdf
This website would like to remind you: Your browser (Apple Safari 4) is out of date. Update your browser for more × security, comfort and the best experience on this site. Encyclopedic Entry ocean gyre For the complete encyclopedic entry with media resources, visit: http://education.nationalgeographic.com/encyclopedia/ocean-gyre/ An ocean gyre is a large system of circular ocean currents formed by global wind patterns and forces created by Earth’s rotation. The movement of the world’s major ocean gyres helps drive the “ocean conveyor belt.” The ocean conveyor belt circulates ocean water around the entire planet. Also known as thermohaline circulation, the ocean conveyor belt is essential for regulating temperature, salinity and nutrient flow throughout the ocean. How a Gyre Forms Three forces cause the circulation of a gyre: global wind patterns, Earth’s rotation, and Earth’s landmasses. Wind drags on the ocean surface, causing water to move in the direction the wind is blowing. The Earth’s rotation deflects, or changes the direction of, these wind-driven currents. This deflection is a part of the Coriolis effect. The Coriolis effect shifts surface currents by angles of about 45 degrees. In the Northern Hemisphere, ocean currents are deflected to the right, in a clockwise motion. In the Southern Hemisphere, ocean currents are pushed to the left, in a counterclockwise motion. Beneath surface currents of the gyre, the Coriolis effect results in what is called an Ekman spiral. While surface currents are deflected by about 45 degrees, each deeper layer in the water column is deflected slightly less. -

Homework 6: Ocean Currents
October 2010 MAR 110 HW6 Ocean Currents 1 Homework 6: Ocean Currents 6-1. OCEAN CURRENTS Ocean currents are water motions induced by winds, tidal forces, and/or density differences with adjacent water masses. Thermohaline currents are generated by under- surface temperature- and salinity-related water mass density differences.. The major oceanic thermohaline circulation system – the so-called conveyer belt- originates with temperature-induced density increases and sinking in the North Atlantic polar regions. The deep current system distributes these cold, dense waters from the polar and subpolar regions toward the Southern Ocean polar region form where they are directed to the other ocean basins and eventual upwelling. Other smaller-scale forms of thermohaline circulation occur in marginal semi-isolated seas, where winter surface cooling and highly saline water inflows cause sinking and water mass formation; and in estuaries, where fresh river water inflows mix with saltier coastal sea water. Wind-driven currents are horizontal motions in the upper layer of the ocean that are primarily driven by the winds and tidal forces. The global winds drive surface current gyres in the major ocean basins. Both thermohaline and wind-driven currents are affected by Earth rotation in ways considered next. 6-2. CORIOLIS EFFECT The Coriolis Effect as it relates to the Earth refers to the deflection of an object from a straight path as observed by an observer on the Earth (or Earth observer). The Earth rotates counterclockwise (CCW) (see Figure 6-1) as viewed by an observer on a point above the North Pole – say the North Star. -

Chlorophyll a Variabliity Due to Large-Scale North Atlantic
CHLOROPHYLL A VARIABILITY DUE TO LARGE-SCALE NORTH ATLANTIC CIRCULATION CHANGES By Alexis L. Santos A thesis submitted in partial fulfillment of the requirements for the degree of Masters of Science Atmospheric and Oceanic Sciences at the University of Wisconsin – Madison May 2014 i Chlorophyll a Variability Due to Large-Scale North Atlantic Circulation Changes Alexis L. Santos Under the supervision of Dr. Galen A. McKinley ABSTRACT The North Atlantic Ocean is one of the strongest oceanic carbon sinks and is heavily influenced by biological uptake. Satellite-observed chlorophyll concentrations, a proxy for phytoplankton biomass, declined by 14% in the North Atlantic inter-gyre region (defined in this study as 40-60 °N, 20-40 °W) over 1998-2006. This study examines the drivers behind satellite chlorophyll a concentration changes over 1998-2006 in this North Atlantic inter-gyre region using a regional biogeochemical model of the North Atlantic basin. Light impacts phytoplankton growth seasonally, but nitrate concentrations drive chlorophyll concentrations on interannual timescales. A nitrate budget of the inter-gyre region finds that along-isopycnal horizontal mixing dominates on the mean and is also a first-order control of interannual variability from 1998-2000 to 2004-2006. Horizontal advection of nutrients is on the same order of magnitude as vertical nitrate advection, and horizontal advection is dominated by advection of nitrate by the North Atlantic nutrient stream. All nitrate transfer processes weaken over time, which is connected -

Regulation of the Phytoplankton Heme B Iron Pool During the North Atlantic Spring Bloom Evangelia Louropoulou, Martha Gledhill, Thomas Browning, Dhwani K
Regulation of the Phytoplankton Heme b Iron Pool During the North Atlantic Spring Bloom Evangelia Louropoulou, Martha Gledhill, Thomas Browning, Dhwani K. Desai, Jan-Lukas Menzel Barraqueta, Manon Tonnard, Géraldine Sarthou, Hélène Planquette, Andrew Bowie, Ruth Schmitz, et al. To cite this version: Evangelia Louropoulou, Martha Gledhill, Thomas Browning, Dhwani K. Desai, Jan-Lukas Menzel Bar- raqueta, et al.. Regulation of the Phytoplankton Heme b Iron Pool During the North Atlantic Spring Bloom. Frontiers in Microbiology, Frontiers Media, 2019, 10, pp.1566. 10.3389/fmicb.2019.01566. hal-02322547 HAL Id: hal-02322547 https://hal.archives-ouvertes.fr/hal-02322547 Submitted on 16 Jun 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. fmicb-10-01566 July 11, 2019 Time: 12:11 # 1 ORIGINAL RESEARCH published: 11 July 2019 doi: 10.3389/fmicb.2019.01566 Regulation of the Phytoplankton Heme b Iron Pool During the North Atlantic Spring Bloom Evangelia Louropoulou1,2*, Martha Gledhill1, Thomas J. Browning1, Dhwani K. Desai3, Jan-Lukas Menzel Barraqueta1,4, Manon Tonnard5,6,7, Géraldine Sarthou5, Hélène Planquette5, Andrew R. Bowie6,7, Ruth A. Schmitz2, Julie LaRoche3 and Eric P. -

Impacts of the North Atlantic Gyre Circulation on Holocene Climate Off Northwest Africa
Impacts of the North Atlantic gyre circulation on Holocene climate off northwest Africa Jung-Hyun Kim*† Universität Bremen, FB 5 Geowissenschaften, Klagenfurter Straße, D-28359 Bremen, Germany Helge Meggers* Norel Rimbu* Alfred Wegener Institute for Polar and Marine Research, Bussestrasse 24, D-27570 Bremerhaven, Germany Gerrit Lohmann* Tim Freudenthal* Universität Bremen, FB 5 Geowissenschaften, Klagenfurter Straße, D-28359 Bremen, Germany Peter J. Müller* Ralph R. Schneider* Christian-Albrechts-Universität zu Kiel, Institut für Geowissenschaften, Ludewig-Meyn-Strasse 10, D-24118 Kiel, Germany ABSTRACT We present well-dated high-resolution Holocene records of sea- surface temperature (SST) and upwelling intensity off northwest (NW) Africa. We identify long-term cooling trends over the Holocene in the subtropical North Atlantic in response to boreal summer insolation. A L warmer pronounced cooling event of ~1 °C ca. 8.5 cal ka indicates a large-scale LC SST reorganization of the ocean current system possibly induced by melt- water from the northern North Atlantic. Our alkenone SST record off NAC Cape Ghir provides strong evidence for the impact of ocean circula- tion changes on subtropical North Atlantic SSTs. It is likely that cold H Colder GS Canary Current SST waters were propagated to the subtropics via the Canary Current in a stronger GeoB 6007-2 way similar to Heinrich events and the Younger Dryas off Cape Blanc. s We fi nd 2–3 k.y. periodic variations in SST and upwelling intensity off wind ade Colder SST gertr NW Africa superimposed on the cooling trend. Such a cycle has been stron documented in various paleoclimate archives in phase with solar forc- +NAO ing. -

Oceanography of the Sargasso Sea: Overview of Scientific Studies M.W
Oceanography of the Sargasso Sea: Overview of Scientific Studies M.W. Lomas, N.R. Bates, K.N. Buck, and A.H. Knap Number 5 Sargasso Sea Alliance Science Report Series When referenced this report should be referred to as: Lomas, M.W., Bates, N.R., Buck, K.N. and A.H. Knap. (eds) 2011a. Oceanography of the Sargasso Sea: Overview of Scientific Studies. Sargasso Sea Alliance Science Report Series, No 5, 64 pp. ISBN 978-0-9847520-7-2 The Sargasso Sea Alliance is led by the Bermuda Government and aims to promote international awareness of the importance of the Sargasso Sea and to mobilise support from a wide variety of national and international organisations, governments, donors and users for protection measures for the Sargasso Sea. Further details: Dr David Freestone, Executive Director, Sargasso Sea Alliance, Suite 300, 1630 Connecticut Avenue NW, Washington D.C., 20009, USA. Email: [email protected] Kate K. Morrison, Deputy Director, at the same address Email: [email protected] The Secretariat of the Sargasso Sea Alliance is hosted by the Washington D.C. Office of the International Union for the Conservation of Nature (IUCN). Website is www.sargassoalliance.org This case is being produced with generous support of donors to the Sargasso Sea Alliance: Ricardo Cisneros, Erik H. Gordon, JM Kaplan Fund, Richard Rockefeller, David E. Shaw, and the Waitt Foundation. Additional support provided by: WWF Sweden and the Pew Environment Group. COVER PHOTO: Taking water samples at Hydrostation S., Tiffany Wardman. ISBN 978-0-9847520-7-2 Oceanography of the Sargasso Sea: Overview of Scientific Studies M.W. -

Ocean Currents
nwhı, currents Ocean Currents and pollutıon Summary Concepts Students will learn what causes ocean currents and how they play a Currents are the oceans role in distributing marine debris and pollution around the world. major means of They will also learn what causes the Coriolis Effect and El Nino. distributing sea water around the globe. With Objectives them they carry pollution and marine • Students will watch an internet video on ocean currents and debris that has a direct complete a worksheet based on what the learned effect on the shores and • Students will practice their understanding of the Coriolis animals of the NWHI . Effect by playing an internet based game on this phenomena • Students will gain an understanding of global impacts that Standards Addressed currents could cause due to global warming SC.8.5.1, 8.8.6, 8.8.7 Materials Duration Computer with Internet connection (audio enabled) 1 1/2 hour Ocean current handout Ocean current worksheet Source Material NOAA Making Connections Students may recall personal experiences when they have Vocabulary experienced an El Nino year and how it differed from a ‘normal’ year Currents weatherwise. They may also realize why certain marine debris that Surface Circulation they have encountered at the beach made its way to Hawaii from Deep Circulation other geographic areas. Gyre Coriolis Effect Teacher Prep for Activity Activity 1: NOAA Internet Video Copy Ocean Current Internet Lesson worksheet so that each student has a copy. Be familiar with Ocean Current Internet Video, lesson 8 http://www.learningdemo.com/noaa/ Activity 2: Coriolis Effect Become familiar with Coriolis Effect game on NOAA website, lesson 8 http://www.learningdemo.com/noaa/ Background Currents are cohesive streams of seawater that circulate through the oceans. -
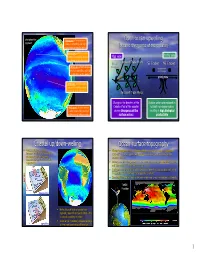
Coastal Up/Down-Welling Ocean Surface Topography
phytoplankton divergence of water masses Open ocean upwelling pigment results in upwelling and high productivity in subpolar waters (oceanic divergence at the equator) convergence of water masses causes near-surface waters Map view Profile view to pile-up in the subtropics Northeast Trade Winds Ekman SE Trades NE Trades divergence of water masses transport winds moving results in upwelling and high into the page productivity at the equator CZ photic zone IT equator convergence of water masses causes near-surface waters to pile-up in the subtropics Southeast Trade Winds Change in the direction of the Surface waters are replaced by divergence of water masses Coriolis effect at the equator nutrient-rich deeper waters results in upwelling and high causes divergence of the resulting in high biological productivity in subpolar waters surface waters productivity Coastal up/down-welling Ocean surface topography • Along coastal areas Ekman • Ekman transport and the Coriolis effect cause surface waters to converge transport can induce downwelling (“pile-up”) in the subtropics, and diverge (move apart) at the equator and in or upwelling by driving water subpolar waters. towards or away from the coast, respectively. • Convergence and divergence of surface water masses create subtle relief (“domes” and “depressions”) on the ocean surface (~1 m). • Gravity acts on the water to pull it downslope (down the pressure gradient), while the Coriolis effect is working in the opposite direction. • Geostrophic flow represents the balance between pressure gradient and Coriolis. NOTE! TOPEX/ Poseidon downwelling • Notice the red (high phytoplankton pigment) area off southwest Africa – this is coastal upwelling in action! • A similar (& important!) process happens upwelling off the west coast of South America 1 Geostrophic flow Global geostrophic flow • Coriolis effect deflects water into the center of the gyres, forming a low mound of water.