Multiphase Fluid Flow Through Porous Media Conductivity And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Seeing Microbes from Space Remote Sensing Is Now a Critical Resource for Tracking Marine Microbial Ecosystem Dynamics and Their Impact on Global Biogeochemical Cycles
Seeing Microbes from Space Remote sensing is now a critical resource for tracking marine microbial ecosystem dynamics and their impact on global biogeochemical cycles Douglas G. Capone and Ajit Subramaniam icrobiologists studying aquatic oceans or other large bodies of water to mean environments are benefiting that they are clean, whereas the brown color of from their use of remote sensing rivers and streams mean they are carrying sus- M technologies. With access to data pended mud or other materials. gathered by remote sensors on This instinctive knowledge forms the basis of aircraft and satellites, microbiologists now can ocean color science. Sunlight that enters the analyze microbial population dynamics with ocean can either be absorbed or scattered back greater breadth and a novel perspective. (Fig. 1). Pure water absorbs most red light but Remote sensing instruments exploit electro- strongly scatters most blue light. Thus, open magnetic radiation to study surface processes on ocean waters with very little material in them earth. Passive sensors use reflected sunlight or appear deep blue, while waters carrying dis- heat being emitted by objects along the earth’s solved organic materials that absorb blue light surface, while active sensors transmit laser or strongly appear brown. Chlorophyll-containing microwaves that are then reflected back to and microalgal assemblages—phytoplankton—ab- recorded by the sensor. Many orbiting satellites sorb both blue and red light, making phyto- currently map ocean properties such as color, plankton-containing waters look green. In addi- surface temperature, height, wind velocities, tion, chlorophyll emits a small fraction of the roughness of the ocean surface, and wave absorbed light at a longer wavelength as red height. -

Programma Dal 22 Al 25 Giugno 2017
IL CORPO UMANO È COMPOSTO AL 70% DA ACQUA IL RESTO È CINEMA AQUA FILM FESTIVAL FILM FILM FILM FESTIVAL FESTIVAL FESTIVAL PROGRAMMA DAL 22 AL 25 GIUGNO 2017 www.aquafilmfestival.org [email protected] aquafilmfestival GIOVEDÌ 22 GIUGNO ORE 15.00 - Spiaggia “LA BIODOLA” - LEZIONE GRATUITA DI GINNASTICA IN ACQUA tenuta dalla Direttrice Artistica Eleonora Vallone, anche Master Trainer all’AQUANIENE e pioniera dell’AcquaGym in Italia, con l’istruttrice dell’Isola d’Elba Silvia Meiattini. CINEMA NELLO SANTI SALA GRANDE ORE 21.30 - ANTEPRIMA DEL FESTIVAL - OMAGGIO ALL’ISOLA D’ELBA Con gli alunni della Scuola Primaria “San Rocco” (Portoferraio) per il progetto “Mi impegno col mare” e “Come eravamo”. Realizzato grazie all’Insegnante Susanna Lemmi e al sostegno del Sindaco Mario Ferrari per le immagini di repertorio storiche. Montaggio di Angelo Del Mastro, testi di Senio Bonini e Michele Baldi. A SEGUIRE - Proiezione del film FUORI CONCORSO “IL BACIO AZZURRO” di P. Tordiglione (IT , 2015) - V.O. - 85 min. Alla presenza dell’attore Sebastiano Somma e dell’attrice Morgana Forcella. VENERDÌ 23 GIUGNO CINEMA NELLO SANTI SALA GRANDE ORE 10.00 - DESK ACCREDITI Distribuzione accrediti giornalisti ed accreditati sul sito di AquaFilmFestival. A SEGUIRE - Coffee Breck offerto da Caffè Corsini. ORE 11.00 - APERTURA FESTIVAL La Direttrice Artistica Eleonora Vallone presenta lo STAFF di AQUAFILMFESTIVAL e la Giuria di AFF composta da: Antonietta De Lillo (marechiarofilm),Simonetta Grechi (Legambiente), Enrico Magrelli (giornalista e critico cinematografico),Filippo Scicchitano (attore), Sara Serraiocco (attrice), Sebastiano Somma (attore) e Cinzia Th. Torrini (regista e scrittrice), alla presenza delle istituzioni di Portoferraio e dei rappresentanti di: Acqua dell’Elba, Visit Elba, Società Albergatori Isola d’Elba e Blue Navy. -

Red Tide Report, 1954
Explanatory Rots The series embodies results of i~vesti~ations,usually of restricted scope, intended to aid or direct mnnagm.ent or utilization practices and as !?ides for udministratiw or legislative action. It C is issued in limited quantities for the official use of Federal, Stnte or cooperating Agencies and in processed form for ecommy and to avoid delay in puhlioation. ? United States Department of the Interior, Douglas McKay, Secretary Fish and Wildlife Service, John L. Farley, Director RED TIDE Progress Report on the Investigations of the Cause of the Mortality of Fish Along the West Coast of Florida Conducted by the U. S. Fish and Wildlife Service and Cooperating Organizations Paul S. Galtsoff Fishery Biologist Fish and Wildlife Service special Scientific Report No. 46 Issued February 1948 Reissued October. 1954 Washington - 1954 I:This report was issued in a limited quantity in 1948, shortly eter the occurrence of red tides off the Florida gulf coast in late 1946 and in 1947. The original supply ras soon exhausted. Public interest in red tides has continued since the earlier outbreaks and has increased recently ag a result of new outbreak@in ths fgll md, winter of 1953-54. Because it contains infonna- tion of general interest, this report is reissued, pending preparation of reports on the latest findings of research on red tides. CONTENTS Page Redtide .............................. 1 Blooming of the sea ........................ 3 . A review of the literature ................... 3 The mortality of fish and the red tide ................9 First outbreak of red tidea November 19b6 .April 19b7 ..... 9 Fish mortality: June 19L7 .................. -

A Beginner's Guide to Water Management — Color
A Beginner’s Guide to Water Management — Color Information Circular 108 Joe Richard Florida LAKEWATCH Department of Fisheries and Aquatic Sciences Institute of Food and Agricultural Sciences University of Florida Gainesville, Florida January 2004 1st Edition This publication was produced by: Florida LAKEWATCH © 2004 University of Florida / Institute of Food and Agricultural Sciences Department of Fisheries and Aquatic Sciences 7922 NW 71st Street Gainesville, FL 32653-3071 Phone: (352) 392-4817 Toll-Free Citizen Hotline: 1-800-LAKEWATch (1-800-525-3928) E-mail: [email protected] Web Address: http://lakewatch.ifas.ufl.edu/ Copies of this document and other information circulars are available for download from the Florida LAKEWATCH website: http://lakewatch.ifas.ufl.edu/LWcirc.html As always, we welcome your questions and comments. A Beginner’s Guide to Water Management — Color Information Circular 108 Florida LAKEWATCH Department of Fisheries and Aquatic Sciences Institute of Food and Agricultural Sciences University of Florida Gainesville, Florida January 2004 1st Edition This publication was produced by: Florida LAKEWATCH © 2004 University of Florida / Institute of Food and Agricultural Sciences Department of Fisheries and Aquatic Sciences 7922 NW 71st Street Gainesville, FL 32653-3071 Phone: (352) 392-4817 Toll-Free Citizen Hotline: 1-800-LAKEWATch (1-800-525-3928) E-mail: [email protected] Web Address: http://lakewatch.ifas.ufl.edu/ Copies of this document and other information circulars are available for download from the Florida LAKEWATCH website: http://lakewatch.ifas.ufl.edu/LWcirc.html As always, we welcome your questions and comments. A Listing of Florida LAKEWATCH Information Circulars Note: For more information related to color in lakes, we recommend that you read Circulars 101, 102 and 103. -

WHY IS BEAR LAKE SO BLUE? and Other Commonly Asked Questions
WHY IS BEAR LAKE SO BLUE? and other commonly asked questions by Jim Davis and Mark Milligan CONTENTS INTRODUCTION...................................................................................................................................2 WHERE IS BEAR LAKE? ...................................................................................................................4 WHY IS THERE A BIG, DEEP LAKE HERE? ..............................................................................6 WHY IS BEAR LAKE SO BLUE? ................................................................................................... 10 WHAT IS THE SHORELINE LIKE? ............................................................................................. 12 WHEN WAS BEAR LAKE DISCOVERED AND SETTLED? ................................................. 15 HOW OLD IS BEAR LAKE? ............................................................................................................16 WHEN DID THE BEAR RIVER AND BEAR LAKE MEET? ................................................ 17 WHAT LIVES IN BEAR LAKE? ..................................................................................................... 20 DOES BEAR LAKE FREEZE? .........................................................................................................24 WHAT IS THE WEATHER AT BEAR LAKE? .......................................................................... 26 WHAT DID BEAR LAKE LOOK LIKE DURING THE ICE AGE? .........................................28 WHAT IS KARST?............................................................................................................................. -

Quantitative Evaluation Method for Landscape Color of Water with Suspended Sediment
water Article Quantitative Evaluation Method for Landscape Color of Water with Suspended Sediment Mao Ye, Ran Li, Weimin Tu, Jialing Liao and Xunchi Pu * State Key Laboratory of Hydraulics and Mountain River Engineering, Sichuan University, Chengdu 610065, China; [email protected] (M.Y.); [email protected] (R.L.); [email protected] (W.T.); [email protected] (J.L.) * Correspondence: [email protected] Received: 31 May 2018; Accepted: 30 July 2018; Published: 6 August 2018 Abstract: Landscape water is an important part of natural landscape, and a reasonable assessment of water landscape color is the basis for scientifically evaluating the quality of water landscape. To evaluate water landscape color with different concentrations of sediment objectively and quantitatively, a method of evaluating water landscape color based on hyperspectral technology is proposed to calculate water landscape color. The color spectrum calculation model of the water landscape color was constructed using the Commission Internationale de L’Eclairage spectrum three stimulus system (CIE-XYZ) calculation method and the response relationship among water reflectance, water depth, and sediment concentration. Under the conditions of eliminating as many external factors as possible, using a hyperspectral instrument to measure the reflectance of sediment and water, the response relationship between water depth and sediment concentration and water reflectance is calculated. Water depth and sediment concentration, which did not appear previously, were verified by experiments that proved the reliability of the water landscape color spectrum calculation model. By using different absolute value of chromatic coordinates in the international CIE-XYZ calculation method, a formula for determining the difference in sediment concentration for water landscape color was defined, and the quantitative evaluation method of landscape color of sand-laden water was established. -

Small Water System Operator Certification Manual
SMALL WATER SYSTEM OPERATOR CERTIFICATION MANUAL For Other-Than-Municipal and Nontransient Noncommunity Public Water Systems Fourth Edition January 2019 Wisconsin Department of Natural Resources Bureau of Drinking Water and Groundwater 101 S. Webster Street PO Box 7921 Madison, WI 53707 The Wisconsin Department of Natural Resources provides equal opportunity in its employment, programs, services and functions under an Affirmative Action Plan. If you have any questions, please write to: Equal Opportunity Office, Department of the Interior, Washington, D.C. 20240. This publication is available in alternative format (large print, Braille, audiotape, etc.) upon request. Please call (608) 266-1054 for more information. PUB DG-068 EXECUTIVE SUMMARY The Federal Safe Drinking Water Act and amendments require all community and nontransient noncommunity public water systems to have an appropriately certified operator managing the system. The intent of this certification requirement is to ensure that the operators of these water systems have the necessary knowledge and training to provide a safe and dependable supply of drinking water to their customers. This manual has been developed for small water system operators to assist in compiling the aforementioned necessary knowledge. The manual is intended to be used as a study guide for the certification exam and as a comprehensive reference manual containing information on all aspects of water system processes and procedures to assist drinking water providers in their day-to-day operations. Small Water System Operator Certification Wisconsin's Small Water System Operator Certification program for “Other-Than- Municipal” and “Nontransient Noncommunity” water systems provides training classes and exams throughout the state. -

Quality Transformation of Dissolved Organic Carbon During Water Transit
Biogeosciences Discuss., https://doi.org/10.5194/bg-2017-279 Manuscript under review for journal Biogeosciences Discussion started: 14 July 2017 c Author(s) 2017. CC BY 4.0 License. Quality transformation of dissolved organic carbon during water transit through lakes: contrasting controls by photochemical and biological processes Martin Berggren1, Marcus Klaus2, Balathandayuthabani Panneer Selvam1, Lena Ström1, Hjalmar 5 Laudon3, Mats Jansson2, Jan Karlsson2 1Department of Physical Geography and Ecosystem Science, Lund University, SE-223 62, Lund, Sweden 2Department of Ecology and Environmental Science, Umeå University, SE-90187, Umeå, Sweden 3Department of Forest Ecology and Management, Swedish University of Agricultural Sciences, SE-90183, Umeå, Sweden Correspondence to: Martin Berggren ([email protected]) 10 Abstract. Dissolved organic carbon (DOC) may be removed, transformed or added during water transit through lakes, resulting in qualitative changes in DOC composition and pigmentation (color). However, the process-based understanding of these changes is incomplete, especially for headwater lakes. We hypothesized that because heterotrophic bacteria preferentially consume non-colored DOC, while photochemical processing remove colored fractions, the overall changes in DOC quality and color (absorbance) upon water passage through a lake depends on the relative importance of these two processes, 15 accordingly. To test this hypothesis we combined laboratory experiments with field studies in nine boreal lakes, assessing both the relative importance of different DOC decay processes (biological or photo-chemical) and the loss of color during water transit time (WTT) through the lakes. We found that photo-chemistry qualitatively dominated the DOC transformation in the epilimnia of relatively clear headwater lakes, resulting in selective losses of colored DOC. -

Density Rainbow
Density Rainbow Learning Objectives: Students investigate the concept of density. GRADE LEVEL SNEAK PEAK inside … ACTIVITY 2–8 Students stack colored liquids in a plastic drinking straw. SCIENCE TOPICS Physical Properties STUDENT SUPPLIES Atoms and Molecules see next page for more supplies Solutions and Mixtures food coloring or Kool AidTM plastic cups sugar PROCESS SKILLS clear straws, etc…. Measuring Predicting ADVANCE PREPARATION Classifying see next page for more details Inferring Fill and label water bottles Label cups of sugar, etc…. GROUP SIZE OPTIONAL EXTRAS 2–4 DEMONSTRATION Modeling the Procedure (p. A - 15) Make the Solutions (p. A - 15) Classroom Density—Constant Volume (p. A - 15) Classroom Density—Constant Mass (p. A - 16) Sink or Float (p. A - 16) EXTENSIONS Mystery Solution (p. A - 23) Calculating Density (p. A - 23) Stack of Liquids (p. A - 24) TIME REQUIRED Advance Preparation Set Up Activity Clean Up 15 minutes 15 minutes 30 minutes 10 minutes Density Rainbow A - 11 Chemistry in the K–8 Classroom Grades 2–8 2007, OMSI SUPPLIES Item Amount Needed tall, clear, narrow plastic cups (9-oz. or 12-oz.) 5 per group masking tape 1 roll per group permanent markers (e.g., Sharpie™) 1 per group granulated sugar 1 cup per group teaspoon measure 1 per group clear plastic drinking straws 1 per student pop-top squeeze bottles (e.g., water or sports drink) 2 per group 16-oz or larger food coloring (red, green, blue, and yellow) OR set of 4 colors per group sugarless Kool Aid™ packets of the same colors a total of 3–4 gallons of access to a sink (cold water works fine) water is needed For Extension or Demonstration supplies, see the corresponding section. -

A Study of Water Quality Handbook
A Study of Water Quality A STUDY OF WATER QUALITY By Dr. Charles E. Renn FOREWORD Even though it is not true that man is losing his race with the growing need for water, it is not debatable that water does hold the key to man’s survival. He must have water to drink, to wash, to grow food, to sustain industrial growth, to support transportation and to provide recreation. Without water, life is barren and hopeless. Men have fought for it, honored it, degraded it and manipulated it. It has always posed problems of too much, too little or too bad. It is a beautiful servant and a tyrannical master. One never misses it until it is not available. In Benjamin Franklin’s wise words, “One does not think of water until the well is dry!” Water is a complex material. After centuries of use, we still do not understand its internal structure. In spite of this ignorance, we can control it for the wise use of man, beast and plant. This booklet helps to clarify our problems, solutions and methods of testing. Abel Wolman Professor Emeritus of Sanitary Engineering and Water Resources The Johns Hopkins University ABOUT THE AUTHOR This text has been prepared by Dr. Charles E. Renn, Professor Emeritus of Environmental Engineering Science, Johns Hopkins University. Dr. Renn holds a B.S. degree in science education from Columbia University, an M.S. degree in science education from New York University, and a Ph.D. in soil microbiology from Rutgers University. Dr. Renn has worked on various biological problems of water supply and waste water disposal with water supply and waste disposal engineers and with most of the larger chemical industries. -

Earth's Oceans
Earth’s Oceans – “The Water Planet” Unit 6 Chapters 22 - 24 Why does the Earth look mostly blue when viewed from space? Oceanography • Oceanography – is the study of the ocean using chemistry, biology, physics, geology, and other sciences. Water Molecule • Water molecule (H20) = 1 Oxygen, 2 Hydrogens • Polarity – uneven distribution of charge between H & O – Hydrogen is positive charge and Oxygen is negative charge = Dipolar molecule • H bonding - strong bond between a positively charged H atom of one molecule with a negatively charged atom of another molecule. – Hydrogen bonds determine the structure of water in solid form. _ – Arrange in “frame” with large spaces O H H + between molecules + (less dense, like ice) _ O H H + + Properties of Water • Oceans cover >70% of Earth’s surface • Density – – Salinity and Temperature affect density of seawater – Ice (solid form of water) is less dense than the liquid form because of the structure • Ice floats on water. – Salt concentration – the more salt the more dense the water is. • (Dead Sea – people float on water http://www.trekearth.com/gallery/Middle_East/Israel/Southern/ because salt water is Beer_Sheva/Dead_Sea/photo1019336.htm more dense). Seawater • Salinity – measure of dissolved salts in water. – Measure by evaporating water Freshwater freezes, leaves and weighing amount of salt salt behind in seawater. left over. – Average salinity = 35 practical salinity units (psu) or 35%. – Based on amount of fresh water entering sea. (so salinity may be different at times – also depends on climate) • Seawater liquid between -2ºC to 100.3ºC (normally water http://tvl1.geo.uc.edu/ice/Image/pretty/467-4.html is a liquid between 0ºC - 100ºC – Throw salt on sidewalk/road to melt ice. -
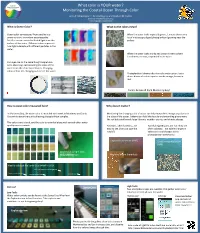
What Color Is YOUR Water? Monitoring the Coastal Ocean Through Color
What color is YOUR water? Monitoring the Coastal Ocean Through Color Anna R. McGaraghan*, Kendra Negrey, and Raphael M. Kudela University of California Santa Cruz *[email protected] What is Ocean Color? What do the colors mean? Ocean color as measured from satellite is a When the water looks especially green, it means there are a prominent tool in modern oceanography. lot of microscopic algae (phytoplankton) growing near the Satellite sensors record reflected light from the surface. surface of the water. Different colors represent how light interacts with different parTcles in the water. When the water looks murky and brown it means there is sediment, or mud, suspended in the water. Our eyes can do the same thing! People have been observing and recording the color of the water from the shore for centuries. Changing colors reflect the changing parTcles in the water. Phytoplankton blooms oNen turn the water green, but a dense bloom of certain species can be orange, brown or red. Colors below all from Monterey Bay! How is ocean color measured here? Why does it maer? In Monterey Bay, the water color is recorded each week in Monterey and Santa Monitoring the changing color of water can help researchers recognize paerns in Cruz while researchers are collecTng phytoplankton samples. the state of the ocean. Paerns can hold the keys to understanding phenomena like red Tdes and harmful algal blooms, weather events, and climate change. This color chart is used, and the color is recorded along with several other water quality measurements. Red Tdes, oNen harmless, are Harmful Algal Blooms are not.