The Removal of Uremic Toxins
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Increase of Urinary Putrescine In3,4-Benzopyrene Carcinogenesis
[CANCER RESEARCH 38, 3509-3511, October 1978] 0008-5472/78/0038-0000$02.00 Increase of Urinary Putrescine in 3,4-Benzopyrene Carcinogenesis and Its Inhibition by Putrescine Keisuke Fujita,1 Toshiharu Nagatsu, Kan Shinpo, Kazuhiro Maruta, Hisahide Takahashi, and Atsushi Sekiya Institute for Comprehensive Medical Science ¡K.F., K. S., K. M.], Research Center for Laboratory Animals ¡H.T.¡,and Department of Pharmacology ¡A.S.¡, Fujita-Gakuen University School of Medicine, Toyoake, Aichi 470-11, Japan, and Laboratory of Cell Physiology, Department of Life Chemistry, Graduate School at Nagatsuta, Tokyo Institute of Technology, Yokohama 227, Japan ¡T.N.¡ ABSTRACT were included. Thirty female BALB/c mice, 19 to 20 weeks old and 25 to 30 g body weight, were given s.c. injections of A significant increase in putrescine was noted in the 2.52 mg of 3,4-benzopyrene in 0.5 ml of tricaprylin (Group urine of mice with experimental s.c. tumors induced by a B) or of 2.52 mg of 3,4-benzopyrene plus 10 mg of putres single injection of 3,4-benzopyrene solution (2.52 mg of cine dissolved in 0.5 ml of tricaprylin (Group B + P). As 3,4-benzopyrene in 0.5 ml of tricaprylin). When 10 mg of controls, 30 mice were given injections of 0.5 ml of tricapry putrescine were added to the 3,4-benzopyrene solution, lin (Group C), and 15 mice received 10 mg of putrescine in the development of tumors was completely inhibited and 0.5 ml of tricaprylin (Group P). -

Laboratory Testing for Chronic Kidney Disease Diagnosis and Management
Test Guide Laboratory Testing for Chronic Kidney Disease Diagnosis and Management Chronic kidney disease is defined as abnormalities of kidney prone to error due to inaccurate timing of blood sampling, structure or function, present for greater than 3 months, incomplete urine collection over 24-hours, or over collection with implications for health.1 Diagnostic criteria include of urine beyond 24-hours.2,3 a decreased glomerular filtration rate (GFR) or presence Given that direct measurement of GFR may be problematic, of 1 or more other markers of kidney damage.1 Markers of eGFR, using either creatinine- or cystatin C-based kidney damage include a histologic abnormality, structural measurements, is most commonly used to diagnose CKD in abnormality, history of kidney transplantation, abnormal urine clinical practice. sediment, tubular disorder-caused electrolyte abnormality, or an increased urinary albumin level (albuminuria). Creatinine-Based eGFR This Test Guide discusses the use of laboratory tests that GFR is typically estimated using the Chronic Kidney Disease 4 may aid in identifying chronic kidney disease and monitoring Epidemiology Collaboration (CKD-EPI) equation. The CKD-EPI and managing disease progression, comorbidities, and equation uses serum-creatinine measurements and the complications. The tests discussed include measurement patient’s age (≥18 years old), sex, and race (African American and estimation of GFR as well as markers of kidney damage. vs non−African American). Creatinine-based eGFR is A list of applicable tests is provided in the Appendix. The recommended by the Kidney Disease Improving Global information is provided for informational purposes only and Outcomes (KDIGO) 2012 international guideline for initial is not intended as medical advice. -

Renal Cortical Mitochondrial Transport of Calcium in Chronic Uremia
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Kidney International, Vol. 34 (1988), pp. 32 7—332 Renal cortical mitochondrial transport of calcium in chronic uremia AvIvA CONFORTY, RUTH SHAINKIN-KESTENBAUM, VARDA SHOSHAN, RINA KOL, JAYSON RAPOPORT, and CIDI0 CHAIM0vITz Departments of Nephrology, Pathology and Biology, Ben-Gurion University of the Negev and Soroka Medical Center, Beer Sheva, Israel Renal cortical mitochondrial transport of calcium in chronic uremia. reduce histologic damage in experimental chronic renal failure Calcium overload of tubular cells may occur in uremia, and may be the in rats [4, 5]. underlying functional abnormality in the continued deterioration of The pathogenic role of cellular overload of calcium as a renal function in chronic renal failure. In order to study this question further, the effect of chronic uremia on the calcium transport properties mediator of cell injury in the kidney has long been recognized and respiratory rates was examined in mitochondria (Mi) isolated from [6—8]. Therefore, it is important to determine if calcium over- the cortex of the remnant kidneys of subtotally nephrectomized rats load of renal cells accompanies the phenomenon of uremic renal (SNX) and sham operated controls (C). Plasma calcium concentration calcification. If, indeed, calcium accumulates in renal cells, it was similar in both groups of rats, but a significant hyperphosphatemia was seen in SNX, 8.60.6 mg%, as compared to 7.20.2 mg% in C could be a contributory factor to the continuing loss of func- (P < 0.001). Mi calcium and phosphate concentrations (nmol/mgtioning nephrons in chronic renal failure. -

The Efficacy and Safety of Six-Weeks of Pre-Workout Supplementation in Resistance Trained Rats
W&M ScholarWorks Undergraduate Honors Theses Theses, Dissertations, & Master Projects 4-2017 The Efficacy and Safety of Six-Weeks of Pre-Workout Supplementation in Resistance Trained Rats Justin P. Canakis College of William and Mary Follow this and additional works at: https://scholarworks.wm.edu/honorstheses Part of the Animal Sciences Commons, Exercise Science Commons, Laboratory and Basic Science Research Commons, and the Other Nutrition Commons Recommended Citation Canakis, Justin P., "The Efficacy and Safety of Six-Weeks of Pre-Workout Supplementation in Resistance Trained Rats" (2017). Undergraduate Honors Theses. Paper 1128. https://scholarworks.wm.edu/honorstheses/1128 This Honors Thesis is brought to you for free and open access by the Theses, Dissertations, & Master Projects at W&M ScholarWorks. It has been accepted for inclusion in Undergraduate Honors Theses by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. 1 2 Title Page……………………………………………………………………………………...…..1 Abstract…………………………………………………………………………………………....5 Acknowledgement……………………………………………………………………….………..6 Background.………………………………………………………………………..……………...7 DSEHA and its Effect on the VMS Industry………………...……………………………7 History of Adverse Side Effects from Pre-Workout Supplements ………….……………8 Ingredient Analysis ………………………………………..……………….……………..……....9 2.5g Beta-Alanine…………………………..……………………………………………..9 1g Creatine Nitrate……………………………...……………………………………..…12 500mg L-Leucine……………………………………………………………………...…16 500mg Agmatine Sulfate……………………….………………………………..………18 -

Profiling of Amino Acids in Urine Samples of Patients Suffering from Inflammatory Bowel Disease by Capillary Electrophoresis-Mass Spectrometry
Article Profiling of Amino Acids in Urine Samples of Patients Suffering from Inflammatory Bowel Disease by Capillary Electrophoresis-Mass Spectrometry Juraj Piestansky 1,2, Dominika Olesova 3, Jaroslav Galba 1, Katarina Marakova 1,2, Vojtech Parrak 3, Peter Secnik 4, Peter Secnik jr. 4, Branislav Kovacech 3, Andrej Kovac 3, Zuzana Zelinkova 5, Peter Mikus 1,2,* 1 Department of Pharmaceutical Analysis and Nuclear Pharmacy, Faculty of Pharmacy, Comenius University in Bratislava, Odbojarov 10, SK-832 32 Bratislava, Slovak Republic; [email protected] (J.P.); [email protected] (J.G.); [email protected] (K.M.); [email protected] (P.M.) 2 Toxicological and Antidoping Center, Faculty of Pharmacy, Comenius University in Bratislava, Odbojárov 10, SK-832 32 Bratislava, Slovak Republic 3 Institute of Neuroimmunology, Slovak Academy of Science, Dubravska cesta 9, SK-845 10, Bratislava, Slovak Republic; [email protected] (D.O.); [email protected] (B.K.); [email protected] (A.K.); [email protected] (V.P.) 4 SK-Lab s.r.o., Partizanska 15, SK-984 01, Lucenec, Slovak Republic; [email protected] (P.S.); [email protected] (P.S. jr.) 5 Department of Gastroenterology, St Michael’s Hospital, Satinskeho 1, SK-811 08 Bratislava, Slovak Republic; [email protected] (Z.Z.) * Correspondence: [email protected]; Tel.: +421-2-50 117 243 (Supplementary Material) Table of content Table S1 Normalized concentrations of amino acids in urine samples from healthy volunteers measured by the CE-MS/MS method. Table S2 Normalized concentrations of amino acids in urine samples from IBD patients undergoing thiopurine treatment measured by the CE-MS/MS method. -

The Efficacy and Safety of Six-Weeks of Pre-Workout Supplementation in Resistance Trained Rats
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by College of William & Mary: W&M Publish W&M ScholarWorks Undergraduate Honors Theses Theses, Dissertations, & Master Projects 4-2017 The Efficacy and Safety of Six-Weeks of Pre-Workout Supplementation in Resistance Trained Rats Justin P. Canakis College of William and Mary Follow this and additional works at: https://scholarworks.wm.edu/honorstheses Part of the Animal Sciences Commons, Exercise Science Commons, Laboratory and Basic Science Research Commons, and the Other Nutrition Commons Recommended Citation Canakis, Justin P., "The Efficacy and Safety of Six-Weeks of Pre-Workout Supplementation in Resistance Trained Rats" (2017). Undergraduate Honors Theses. Paper 1128. https://scholarworks.wm.edu/honorstheses/1128 This Honors Thesis is brought to you for free and open access by the Theses, Dissertations, & Master Projects at W&M ScholarWorks. It has been accepted for inclusion in Undergraduate Honors Theses by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. 1 2 Title Page……………………………………………………………………………………...…..1 Abstract…………………………………………………………………………………………....5 Acknowledgement……………………………………………………………………….………..6 Background.………………………………………………………………………..……………...7 DSEHA and its Effect on the VMS Industry………………...……………………………7 History of Adverse Side Effects from Pre-Workout Supplements ………….……………8 Ingredient Analysis ………………………………………..……………….……………..……....9 2.5g Beta-Alanine…………………………..……………………………………………..9 -

Effects of the Acute Arginine Aspartate Supplement on the Muscular Fatigue
ARTIGO ORIGINAL Efeitos da suplementação aguda de aspartato de arginina na fadiga muscular em voluntários treinados Ricardo Pombo Sales1, Carlos Eduardo César Miné1, Andréia Dellú Franco1, Érika Lima Rodrigues1, Naira Correia Cusma Pelógia2, Renato de Souza e Silva3, José Carlos Cogo1, Rodrigo A.B. Lopes-Martins1, Rodrigo Lazo Osorio1 e Wellington Ribeiro1 RESUMO Palavras-chave: Lactato. Aminoácido. Exaustão. A atividade física influi em mecanismos específicos responsá- Keywords: Lactate. Aminoacid. Exhaustion. veis pela redução da produção de força e conseqüentemente à Palabras-clave: Lactato. Aminoácido. Agotamiento. fadiga. A preocupação em melhorar o desempenho físico tem sido propostos; observamos que estudos dão atenção para reduzir acú- mulos dos metabólitos que diminuem a fadiga durante o exercício cal performance, and we observed that some studies have been físico intenso, usando aminoácidos conhecidos por induzir mudan- focused on the reduction of the metabolites that decrease the ças metabólicas, entre eles a arginina. O presente estudo teve fatigue on intense physical exercising, using aminoacids known como objetivo estudar o efeito da suplementação aguda de aspar- for their properties to induce to metabolic changes, and among tato de arginina em indivíduos sadios treinados submetidos a um these, it is the arginine. The present study had the purpose to protocolo de exaustão em um cicloergômetro. Foram utilizados study the effects of the acute arginine aspartate supplement in 12 indivíduos treinados do sexo masculino, idade de 22,6 ± 3,5 trained healthy individuals submitted to an exhaustion protocol on anos. Realizaram três testes 90 minutos após a administração em ergonomic bicycle. Twelve 22.6 ± 3.5 years old trained individuals dose única do aspartato de arginina ou solução placebo, em um were used in the research. -

Renal Effects of the Serine Protease Inhibitor Aprotinin in Healthy Conscious Mice
www.nature.com/aps ARTICLE OPEN Renal effects of the serine protease inhibitor aprotinin in healthy conscious mice Stefan Wörner1, Bernhard N. Bohnert1,2,3, Matthias Wörn1, Mengyun Xiao1, Andrea Janessa1, Andreas L. Birkenfeld1,2,3, Kerstin Amann4, Christoph Daniel4 and Ferruh Artunc 1,2,3 Treatment with aprotinin, a broad-spectrum serine protease inhibitor with a molecular weight of 6512 Da, was associated with acute kidney injury, which was one of the reasons for withdrawal from the market in 2007. Inhibition of renal serine proteases regulating the epithelial sodium channel ENaC could be a possible mechanism. Herein, we studied the effect of aprotinin in wild- type 129S1/SvImJ mice on sodium handling, tubular function, and integrity under a control and low-salt diet. Mice were studied in metabolic cages, and aprotinin was delivered by subcutaneously implanted sustained release pellets (2 mg/day over 10 days). Mean urinary aprotinin concentration ranged between 642 ± 135 (day 2) and 127 ± 16 (day 8) µg/mL . Aprotinin caused impaired sodium preservation under a low-salt diet while stimulating excessive hyperaldosteronism and unexpectedly, proteolytic activation of ENaC. Aprotinin inhibited proximal tubular function leading to glucosuria and proteinuria. Plasma urea and cystatin C concentration increased significantly under aprotinin treatment. Kidney tissues from aprotinin-treated mice showed accumulation of intracellular aprotinin and expression of the kidney injury molecule 1 (KIM-1). In electron microscopy, electron-dense deposits were observed. There was no evidence for kidney injury in mice treated with a lower aprotinin dose (0.5 mg/day). In conclusion, high doses of aprotinin exert nephrotoxic effects by accumulation in the tubular system of healthy mice, leading to inhibition of proximal tubular 1234567890();,: function and counterregulatory stimulation of ENaC-mediated sodium transport. -

Interpretive Guide
INTERPRETIVE GUIDE Contents INTRODUCTION .........................................................................1 NUTREVAL BIOMARKERS ...........................................................5 Metabolic Analysis Markers ....................................................5 Malabsorption and Dysbiosis Markers .....................................5 Cellular Energy & Mitochondrial Metabolites ..........................6 Neurotransmitter Metabolites ...............................................8 Vitamin Markers ....................................................................9 Toxin & Detoxification Markers ..............................................9 Amino Acids ..........................................................................10 Essential and Metabolic Fatty Acids .........................................13 Cardiovascular Risk ................................................................15 Oxidative Stress Markers ........................................................16 Elemental Markers ................................................................17 Toxic Elements .......................................................................18 INTERPRETATION-AT-A-GLANCE .................................................19 REFERENCES .............................................................................23 INTRODUCTION A shortage of any nutrient can lead to biochemical NutrEval profile evaluates several important biochemical disturbances that affect healthy cellular and tissue pathways to help determine nutrient -

Creatinine Clearance 118 Ml/Min 75- 120
LAB #: U000000-0000-0 CLIENT #: 12345 PATIENT: Sample Patient DOCTOR: ID: PATIENT -S-00716 Doctor's Data, Inc. SEX: Female 3755 Illinois Ave. AGE: 42 St. Charles, IL 60174 !!"#$%&'&'#(!)#$"$'*# RESULTS REFERENCE RESULT / UNIT INTERVAL -2SD -1SD MEAN +1SD +2SD Creatinine Clearance 118 mL/min 75- 120 Urine Creatinine 1300 mg/time 600- 1900 Serum Creatinine 0.77 mg/dL 0.6- 1.3 INFORMATION Creatinine Clearance is the most widely used test for estimating glomerular filtration rate (GFR). Creatinine, a breakdown product of muscle creatine, is present in relatively stable levels in serum. It is filtered by the glomeruli and not reabsorbed by the tubules. Changes in renal function are reflected in levels of serum urea and creatinine. It is not uncommon for elderly patients, and those with heavy metal toxicity to have mild to moderate impairment of renal function. Renal disease is asymptomatic in most cases until late in its clinical course. Safe chelation therapy is highly dependent upon the adequacy of renal function. Excessive mobilization of toxic metals to poorly functioning kidneys may result in renal complications. It is advised that creatinine clearance be monitored prior to and throughout chelation therapy. Interpretive guidelines: 100 mL/min or higher usually indicates normal renal function. 50 mL/min or below is indicative of impaired kidney function. 30 mL/min or below is indicative of symptomatic renal failure. Exercise may cause increased clearance. Inaccurate results may be caused by failure to accurately follow the specimen collection instructions. The calculation for corrected creatinine clearance in mL/min: = Urine volume per minute x urine creatinine ÷ Serum creatinine x 1.73/body surface area References: 1. -
Understanding Your Lab Results: Comprehensive Metabolic Screening Panel
Understanding Your Lab Results: Comprehensive Metabolic Screening Panel CHOLESTEROL and TRIGLYCERIDES are fats necessary for normal cell function. However, elevated levels of these fats have been associated with an increased risk of developing coronary disease, arteriosclerosis, and heart attack. A patient’s dietary status, medications, presence of illness, lifestyle, and family history may represent factors influencing cholesterol levels. The signifi- cance of cholesterol levels should be determined within the context of each individual patient. If your cholesterol level is 200 mg/dl or greater, please consult your physician. Triglyceride levels greater than 150 mg/dl, in a true fasting specimen, are considered elevated. In this case, please consult your physician. HDL (High-Density Lipoprotein) CHOLESTEROL, commonly known as the “GOOD” cholesterol, picks up cholesterol and transports it for removal from the body. The higher the HDL value, the lower the risk of developing coronary disease, arterioscle- rosis, and heart attack. LDL (Low-Density Lipoprotein) CHOLESTEROL, commonly known as the “BAD” cholesterol, picks up cholesterol and transports it to the cells of the body for storage. Desirable LDL levels are less than 130 mg/dl. The higher the LDL value, the greater the risk of developing coronary disease, arteriosclerosis, and heart attack. CHOLESTEROL/HDL RATIO is a calculation used to predict an increased or decreased risk of cardiovascular disease relative to a normal. The higher the ratio, the greater the risk of developing coronary disease, arteriosclerosis, and heart attack. GLUCOSE, commonly called a blood sugar, is the transport form of carbohydrates in the body as they move to storage or to utiliza- tion. -
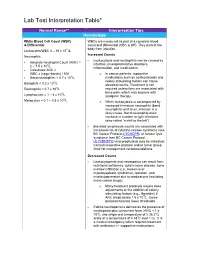
Lab Test Interpretation Table*
Lab Test Interpretation Table* Normal Range** Interpretation Tips Hematology White Blood Cell Count (WBC) WBCs are measured as part of a complete blood & Differential count and differential (CBC & diff). They protect the body from infection. Leukocytes/WBC 4 – 10 x 109 /L Increased Counts Neutrophils - Leukocytosis and neutrophilia can be caused by Absolute Neutrophil Count (ANC) = - infection, myeloproliferative disorders, 2 – 7.5 x 109/L inflammation, and medications. - Calculated ANC = WBC x (segs+bands) / 100 o In cancer patients, supportive - Band neutrophils: < 0.7 x 109/L medications such as corticosteroids and 9 colony stimulating factors can cause Basophils < 0.2 x 10 /L elevated counts. Treatment is not Eosinophils < 0.7 x 109/L required unless they are associated with 9 bone pain, which may improve with Lymphocytes = 1 – 4 x 10 /L analgesic therapy. 9 Monocytes = 0.1 – 0.8 x 10 /L o When leukocytosis is accompanied by increased immature neutrophils (band neutrophils) and fever, infection is a likely cause. Band neutrophils often increase in number to fight infections (also called “a shift to the left”). - Elevated lymphocyte counts are associated with increased risk of cytokine-release syndrome (see BC Cancer Protocol LYCHOPR) or tumour lysis syndrome (see BC Cancer Protocol ULYVENETO) and prophylaxis may be indicated. Consult respective protocol and/or tumor group chair for management recommendations. Decreased Counts - Leukocytopenia and neutropenia can result from nutritional deficiency, autoimmune disease, bone marrow infiltration (i.e., leukemia or myelodysplastic syndrome), radiation, and myelosuppression due to medications (including many cancer drugs). o Many treatment protocols require dose adjustments or the addition of colony stimulating factors (e.g., filgrastim) if ANC drops below 1.5 x 109/L.