From Field to Fat
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Coexistence Between Cyphomyrmex Ants and Dominant Populations Of
Behavioural Processes 74 (2007) 93–96 Coexistence between Cyphomyrmex ants and dominant populations of Wasmannia auropunctata Julien Grangier ∗, Julien Le Breton, Alain Dejean, Jer´ omeˆ Orivel Laboratoire Evolution et Diversit´e Biologique, UMR-CNRS 5174, Universit´e Toulouse III, 31062 Toulouse Cedex 9, France Received 16 September 2005; received in revised form 17 October 2006; accepted 17 October 2006 Abstract The little fire ant Wasmannia auropunctata is able to develop highly dominant populations in disturbed areas of its native range, with a resulting negative impact on ant diversity. We report here on the tolerance of such populations towards several fungus-growing ants of the genus Cyphomyrmex (rimosus complex) in French Guiana. This tolerance is surprising given the usually high interspecific aggressiveness of W. auropunctata when dominant. In order to understand the mechanisms behind such proximity, aggressiveness tests were performed between workers of the different species. These behavioural assays revealed a great passivity in Cyphomyrmex workers during confrontations with W. auropunctata workers. We also found that the aggressiveness between W. auropunctata and two Cyphomyrmex species was more intense between distant nests than between adjacent ones. This dear–enemy phenomenon may result from a process of habituation contributing to the ants’ ability to coexist over the long term. © 2006 Elsevier B.V. All rights reserved. Keywords: Aggressiveness; Cyphomyrmex; Dear–enemy phenomenon; Habituation; Wasmannia auropunctata 1. Introduction present study, we evaluate the possible mechanisms behind this coexistence. Native to the Neotropics, the little fire ant Wasmannia aurop- unctata (Roger) (Myrmicinae) is one of the most problematic 2. Materials and methods invasive ants known, with accompanying ecological and eco- nomical consequences (Holway et al., 2002). -
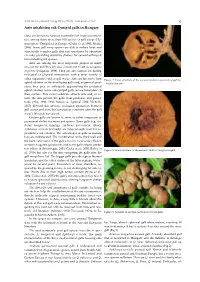
Ants Inhabiting Oak Cynipid Galls in Hungary
North-Western Journal of Zoology 2020, vol.16 (1) - Correspondence: Notes 95 Ants inhabiting oak Cynipid galls in Hungary Oaks are known to harbour extremely rich insect communi- ties, among them more than 100 species of gall wasps (Hy- menoptera: Cynipidae) in Europe (Csóka et al. 2005, Melika 2006). Some gall wasp species are able to induce large and structurally complex galls that can sometimes be abundant on oaks, providing attractive shelters for several arthropod taxa including ant species. Ants are among the most important players in many ecosystems and they are also considered to act as ecosystem engineers (Folgarait, 1998). They are also famous for having ecological or physical interactions with a great variety of other organisms, such as gall wasps. Ants are known to tend Figure 1. Inner structure of the asexual Andricus quercustozae gall in- aphid colonies on the developing galls and, as general pred- habited by ants. ators, they prey on arthropods approaching the protected aphid colonies. Some oak cynipid galls secrete honeydew on their surface. This sweet substrate attracts ants and, in re- turn, the ants protect the galls from predators and parasi- toids (Abe, 1988, 1992; Inouye & Agrawal 2004; Nicholls, 2017). Beyond this obvious ecological interaction between gall wasps and ants, this association continues after the gall wasp’s life cycle has ceased. Certain galls are known to serve as either temporary or permanent shelter for many ant species. Some galls (e.g. An- dricus hungaricus (Hartig), Andricus quercustozae (Bosc), Aphelonyx cerricola (Giraud)) are large enough even for re- productive ant colonies. The advantages of galls as nesting logs are multifaceted. -

Environmental Determinants of Leaf Litter Ant Community Composition
Environmental determinants of leaf litter ant community composition along an elevational gradient Mélanie Fichaux, Jason Vleminckx, Elodie Alice Courtois, Jacques Delabie, Jordan Galli, Shengli Tao, Nicolas Labrière, Jérôme Chave, Christopher Baraloto, Jérôme Orivel To cite this version: Mélanie Fichaux, Jason Vleminckx, Elodie Alice Courtois, Jacques Delabie, Jordan Galli, et al.. Environmental determinants of leaf litter ant community composition along an elevational gradient. Biotropica, Wiley, 2020, 10.1111/btp.12849. hal-03001673 HAL Id: hal-03001673 https://hal.archives-ouvertes.fr/hal-03001673 Submitted on 12 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. BIOTROPICA Environmental determinants of leaf-litter ant community composition along an elevational gradient ForJournal: PeerBiotropica Review Only Manuscript ID BITR-19-276.R2 Manuscript Type: Original Article Date Submitted by the 20-May-2020 Author: Complete List of Authors: Fichaux, Mélanie; CNRS, UMR Ecologie des Forêts de Guyane (EcoFoG), AgroParisTech, CIRAD, INRA, Université -

Check List 8(4): 722–730, 2012 © 2012 Check List and Authors Chec List ISSN 1809-127X (Available at Journal of Species Lists and Distribution
Check List 8(4): 722–730, 2012 © 2012 Check List and Authors Chec List ISSN 1809-127X (available at www.checklist.org.br) Journal of species lists and distribution Check list of ground-dwelling ants (Hymenoptera: PECIES S Formicidae) of the eastern Acre, Amazon, Brazil OF Patrícia Nakayama Miranda 1,2*, Marco Antônio Oliveira 3, Fabricio Beggiato Baccaro 4, Elder Ferreira ISTS 1 5,6 L Morato and Jacques Hubert Charles Delabie 1 Universidade Federal do Acre, Centro de Ciências Biológicas e da Natureza. BR 364 – Km 4 – Distrito Industrial. CEP 69915-900. Rio Branco, AC, Brazil. 2 Instituo Federal do Acre, Campus Rio Branco. Avenida Brasil 920, Bairro Xavier Maia. CEP 69903-062. Rio Branco, AC, Brazil. 3 Universidade Federal de Viçosa, Campus Florestal. Rodovia LMG 818, Km 6. CEP 35690-000. Florestal, MG, Brazil. 4 Instituto Nacional de Pesquisas da Amazônia, Programa de Pós-graduação em Ecologia. CP 478. CEP 69083-670. Manaus, AM, Brazil. 5 Comissão Executiva do Plano da Lavoura Cacaueira, Centro de Pesquisas do Cacau, Laboratório de Mirmecologia – CEPEC/CEPLAC. Caixa Postal 07. CEP 45600-970. Itabuna, BA, Brazil. 6 Universidade Estadual de Santa Cruz. CEP 45650-000. Ilhéus, BA, Brazil. * Corresponding author. E-mail: [email protected] Abstract: The ant fauna of state of Acre, Brazilian Amazon, is poorly known. The aim of this study was to compile the species sampled in different areas in the State of Acre. An inventory was carried out in pristine forest in the municipality of Xapuri. This list was complemented with the information of a previous inventory carried out in a forest fragment in the municipality of Senador Guiomard and with a list of species deposited at the Entomological Collection of National Institute of Amazonian Research– INPA. -

TESE DE DOUTORADO Interações Formiga-Planta Nos Campos Rupestres: Diversidade, Estrutura E Dinâmica Temporal FERNANDA VIEIRA
UNIVERSIDADE FEDERAL DE MINAS GERAIS Instituto de Ciências Biológicas Programa de Pós-Graduação em Ecologia, Conservação e Manejo da Vida Silvestre ______________________________________________________________________ TESE DE DOUTORADO Interações formiga-planta nos campos rupestres: diversidade, estrutura e dinâmica temporal FERNANDA VIEIRA DA COSTA BELO HORIZONTE 2016 FERNANDA VIEIRA DA COSTA Interações formiga-planta nos campos rupestres: diversidade, estrutura e dinâmica temporal Tese apresentada ao Programa de Pós- Graduação em Ecologia, Conservação e Manejo da Vida Silvestre da Universidade Federal de Minas Gerais, como requisito parcial para obtenção do título de Doutora em Ecologia, Conservação e Manejo da Vida Silvestre. Orientador: Dr. Frederico de Siqueira Neves Coorientadores: Dr. Marco Aurelio Ribeiro de Mello & Dr. Tadeu José de Abreu Guerra BELO HORIZONTE 2016 2 3 Agradecimentos À Universidade Federal de Minas Gerais (UFMG) e ao Programa de Pós-Graduação em Ecologia, Conservação e Manejo da Vida Silvestre (ECMVS), pela oportunidade, apoio e excelente formação acadêmica. Especialmente aos professores Frederico Neves, Marco Mello, Adriano Paglia e Fernando Silveira pelos ensinamentos e conselhos transmitidos. Agradeço também aos secretários Frederico Teixeira e Cristiane por todo auxílio com as burocracias, que facilitaram muito pra que essa caminhada fosse mais tranquila. À Fundação CAPES pela concessão da bolsa durante o período do doutorado realizado no Brasil. Ao Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) e ao Deutscher Akademischer Austauschdiens (DAAD) pela oportunidade de realização do doutorado sanduíche na Alemanha e concessão da bolsa durante o intercâmbio. Ao CNPq (Chamada Universal, Processo 478565/2012-7) e ao Projeto de Pesquisas Ecológicas de Longa Duração (PELD – Campos Rupestres da Serra do Cipó) pelo apoio financeiro e logístico. -

REVISIÓN TAXONÓMICA DE LAS HORMIGAS Tapinoma Förster (HYMENOPTERA: FORMICIDAE: DOLICHODERINAE) EN LA REGIÓN NEOTROPICAL
UNIVERSIDAD CENTRAL DE VENEZUELA FACULTAD DE CIENCIAS POSTGRADO EN ZOOLOGÍA REVISIÓN TAXONÓMICA DE LAS HORMIGAS Tapinoma Förster (HYMENOPTERA: FORMICIDAE: DOLICHODERINAE) EN LA REGIÓN NEOTROPICAL Tesis Doctoral presentado ante la ilustre Universidad Central de Venezuela por el Ldo. Roberto José Guerrero Flórez, para optar al título de Doctor en Ciencias Tutor (es): Juan Carlos Navarro, Ph. D. Fernando Fernández, Ph. D (ICN-UN, Colombia) Caracas – Venezuela Octubre de 2017 2 3 RESUMEN Las hormigas del género Tapinoma son un elemento conspicuo adentro de la subfamilia Dolichoderinae. Estas hormigas son cosmopolitas, exhibiendo una mayor riqueza de especies en las regiones paleotropicales, no obstante, la región Neotropical alberga una fauna considerable de especies de Tapinoma. Por primera vez y tomando en consideración las especies de la región Neotropical, se revisan taxonómicamente las especies del género Tapinoma. El análisis morfológico, datos de distribución y en algunos casos la integración de información ecológica, respaldan la delimitación de 10 especies distribuidas en cuatro grupos de especies (Grupo Litorale, Grupo Melanocephalum, Grupo Ramulorum y Grupo Sessile), de los cuales Litorale y Ramulorum albergan el mayor número de especies Neotropicales. El esquema taxonómico es el siguiente: Tapinoma amazonae Wheeler, W.M. 1934, Tapinoma atriceps Emery, 1888 (=Tapinoma atriceps breviscapus Forel, 1908), Tapinoma inrectum Forel, 1908 (estatus revisado y revivido), T. litorae Wheeler, 1905 (=litorale cubaensis Wheeler, W.M. 1913, nuevo sinónimo; =panamense Wheeler, W.M. 1934, nuevo sinónimo), T. melanocephalum (Fabricius, 1793) (=T. luffae (Kuriam 1955), nuevo sinónimo; =T. melanocephalum coronatum Forel, 1908, nuevo sinónimo; =T. melanocephalum malesianum Forel, 1913, nuevo sinónimo), T. opacum Wheeler, W.M. & Mann, 1914, T. -

How Does a Strip of Clearing Affect the Forest Community of Ants (Hymenoptera: Formicidae)?
Fragmenta Faunistica 52 (2): 125-141, 2009 PL ISSN 0015-9301 O MUSEUM AND INSTITUTE OF ZOOLOGY PAS How does a strip of clearing affect the forest community of ants (Hymenoptera: Formicidae)? Hanna B a b ik *, Wojciech CZECHOWSKI*, Tomasz WŁODARCZYK** and Maria STERZYŃSKA* * Museum and Institute of Zoology PAS, Laboratory of Social and Myrmecophilous Insects, Wilcza St 64, 00-679 Warszawa, Poland; e-mails: [email protected], [email protected], [email protected] **University o f Białystok, Department o f Invertebrate Zoology, Świerkowa St 20B, 15-950 Białystok, Poland; e-mail: t. wlodar@uwb. edu.pl Abstract: Tlie species composition, nest density, structure and ecological profile of an ant community were studied within a transect encompassing the forest interior, forest edge and a belt-shaped clearing in a moist mixed pine forest habitat (Querco roboris-Pinetum) in the Kampinos Forest (Central Poland) in the context of direct and indirect human impact and the bioindicator importance of ants. Altogether, 19 ant species were found; the most abundant ones (in respect of number of nests) in the entire habitat under study were Temnothorax crassispinus (Karav.) and Myrmica rubra (L.). All analysed parameters of individual subcommunities, except for nest density (highest on the forest edge, lowest in the cleared belt), showed a gradient pattern of variability, with species richness and the index of general diversity increasing and the dominance index decreasing within the transect from the forest interior to the cleared belt. Differences between the two subcommunities from the forested area (forest interior and forest edge), both highly dominated by T. -

Recerca I Territori V12 B (002)(1).Pdf
Butterfly and moths in l’Empordà and their response to global change Recerca i territori Volume 12 NUMBER 12 / SEPTEMBER 2020 Edition Graphic design Càtedra d’Ecosistemes Litorals Mediterranis Mostra Comunicació Parc Natural del Montgrí, les Illes Medes i el Baix Ter Museu de la Mediterrània Printing Gràfiques Agustí Coordinadors of the volume Constantí Stefanescu, Tristan Lafranchis ISSN: 2013-5939 Dipòsit legal: GI 896-2020 “Recerca i Territori” Collection Coordinator Printed on recycled paper Cyclus print Xavier Quintana With the support of: Summary Foreword ......................................................................................................................................................................................................... 7 Xavier Quintana Butterflies of the Montgrí-Baix Ter region ................................................................................................................. 11 Tristan Lafranchis Moths of the Montgrí-Baix Ter region ............................................................................................................................31 Tristan Lafranchis The dispersion of Lepidoptera in the Montgrí-Baix Ter region ...........................................................51 Tristan Lafranchis Three decades of butterfly monitoring at El Cortalet ...................................................................................69 (Aiguamolls de l’Empordà Natural Park) Constantí Stefanescu Effects of abandonment and restoration in Mediterranean meadows .......................................87 -
Arkansas Academy of Science
Journal of the CODEN: AKASO ISBN: 0097-4374 ARKANSAS ACADEMY OF SCIENCE VOLUME 61 2007 Library Rate ARKANSAS ACADEMY OF SCIENCE ARKANSAS TECH UNIVERSITY DEPARTMENT OF PHYSICAL SCIENCES 1701 N. BOULDER RUSSELLVILLE. AR 72801-2222 Arkansas Academy ofScience, Dept. of Physical Sciences, Arkansas Tech University PAST PRESIDENTS OF THE ARKANSAS ACADEMY OF SCIENCE Charles Brookover, 1917 C. E. Hoffman, 1959 Paul Sharrah, 1984 Dwight M. Moore, 1932-33, 64 N. D. Buffaloe, 1960 William L. Evans, 1985 Flora Haas, 1934 H. L. Bogan, 1961 Gary Heidt, 1986 H. H. Hyman, 1935 Trumann McEver, 1962 Edmond Bacon, 1987 L. B. Ham, 1936 Robert Shideler, 1963 Gary Tucker, 1988 W. C. Muon, 1937 L. F. Bailey, 1965 David Chittenden, 1989 M. J. McHenry, 1938 James H. Fribourgh, 1966 Richard K. Speairs, Jr. 1990 T. L. Smith, 1939 Howard Moore, 1967 Robert Watson, 1991 P. G. Horton, 1940 John J. Chapman, 1968 Michael W. Rapp, 1992 I. A. Willis, 1941-42 Arthur Fry, 1969 Arthur A. Johnson, 1993 L. B. Roberts, 1943-44 M. L. Lawson, 1970 George Harp, 1994 JeffBanks, 1945 R. T. Kirkwood, 1971 James Peck, 1995 H. L. Winburn, 1946-47 George E. Templeton, 1972 Peggy R. Dorris, 1996 E. A. Provine, 1948 E. B. Wittlake, 1973 Richard Kluender, 1997 G. V. Robinette, 1949 Clark McCarty, 1974 James Daly, 1998 John R. Totter, 1950 Edward Dale, 1975 Rose McConnell, 1999 R. H. Austin, 1951 Joe Guenter, 1976 Mostafa Hemmati, 2000 E. A. Spessard, 1952 Jewel Moore, 1977 Mark Draganjac, 2001 Delbert Swartz, 1953 Joe Nix, 1978 John Rickett, 2002 Z. -

Akes an Ant an Ant? Are Insects, and Insects Are Arth Ropods: Invertebrates (Animals With
~ . r. workers will begin to produce eggs if the queen dies. Because ~ eggs are unfertilized, they usually develop into males (see the discus : ~ iaplodiploidy and the evolution of eusociality later in this chapter). =- cases, however, workers can produce new queens either from un ze eggs (parthenogenetically) or after mating with a male ant. -;c. ant colony will continue to grow in size and add workers, but at -: :;oint it becomes mature and will begin sexual reproduction by pro· . ~ -irgin queens and males. Many specie s produce males and repro 0 _ " females just before the nuptial flight . Others produce males and ---: : ._ tive fem ales that stay in the nest for a long time before the nuptial :- ~. Our largest carpenter ant, Camponotus herculeanus, produces males _ . -:= 'n queens in late summer. They are groomed and fed by workers :;' 0 it the fall and winter before they emerge from the colonies for their ;;. ights in the spring. Fin ally, some species, including Monomoriurn : .:5 and Myrmica rubra, have large colonies with multiple que ens that .~ ..ew colonies asexually by fragmenting the original colony. However, _ --' e polygynous (literally, many queens) and polydomous (literally, uses, referring to their many nests) ants eventually go through a -">O=- r' sexual reproduction in which males and new queens are produced. ~ :- . ant colony thus functions as a highly social, organ ized "super _ _ " 1." The queens and mo st workers are safely hidden below ground : : ~ - ed within the interstices of rotting wood. But for the ant workers ~ '_i S ' go out and forage for food for the colony,'life above ground is - =- . -

Bee Viruses: Routes of Infection in Hymenoptera
fmicb-11-00943 May 27, 2020 Time: 14:39 # 1 REVIEW published: 28 May 2020 doi: 10.3389/fmicb.2020.00943 Bee Viruses: Routes of Infection in Hymenoptera Orlando Yañez1,2*, Niels Piot3, Anne Dalmon4, Joachim R. de Miranda5, Panuwan Chantawannakul6,7, Delphine Panziera8,9, Esmaeil Amiri10,11, Guy Smagghe3, Declan Schroeder12,13 and Nor Chejanovsky14* 1 Institute of Bee Health, Vetsuisse Faculty, University of Bern, Bern, Switzerland, 2 Agroscope, Swiss Bee Research Centre, Bern, Switzerland, 3 Laboratory of Agrozoology, Department of Plants and Crops, Faculty of Bioscience Engineering, Ghent University, Ghent, Belgium, 4 INRAE, Unité de Recherche Abeilles et Environnement, Avignon, France, 5 Department of Ecology, Swedish University of Agricultural Sciences, Uppsala, Sweden, 6 Environmental Science Research Center, Faculty of Science, Chiang Mai University, Chiang Mai, Thailand, 7 Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai, Thailand, 8 General Zoology, Institute for Biology, Martin-Luther-University of Halle-Wittenberg, Halle (Saale), Germany, 9 Halle-Jena-Leipzig, German Centre for Integrative Biodiversity Research (iDiv), Leipzig, Germany, 10 Department of Biology, University of North Carolina at Greensboro, Greensboro, NC, United States, 11 Department Edited by: of Entomology and Plant Pathology, North Carolina State University, Raleigh, NC, United States, 12 Department of Veterinary Akio Adachi, Population Medicine, College of Veterinary Medicine, University of Minnesota, Saint Paul, MN, United States, -

Sistemática Y Ecología De Las Hormigas Predadoras (Formicidae: Ponerinae) De La Argentina
UNIVERSIDAD DE BUENOS AIRES Facultad de Ciencias Exactas y Naturales Sistemática y ecología de las hormigas predadoras (Formicidae: Ponerinae) de la Argentina Tesis presentada para optar al título de Doctor de la Universidad de Buenos Aires en el área CIENCIAS BIOLÓGICAS PRISCILA ELENA HANISCH Directores de tesis: Dr. Andrew Suarez y Dr. Pablo L. Tubaro Consejero de estudios: Dr. Daniel Roccatagliata Lugar de trabajo: División de Ornitología, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia” Buenos Aires, Marzo 2018 Fecha de defensa: 27 de Marzo de 2018 Sistemática y ecología de las hormigas predadoras (Formicidae: Ponerinae) de la Argentina Resumen Las hormigas son uno de los grupos de insectos más abundantes en los ecosistemas terrestres, siendo sus actividades, muy importantes para el ecosistema. En esta tesis se estudiaron de forma integral la sistemática y ecología de una subfamilia de hormigas, las ponerinas. Esta subfamilia predomina en regiones tropicales y neotropicales, estando presente en Argentina desde el norte hasta la provincia de Buenos Aires. Se utilizó un enfoque integrador, combinando análisis genéticos con morfológicos para estudiar su diversidad, en combinación con estudios ecológicos y comportamentales para estudiar la dominancia, estructura de la comunidad y posición trófica de las Ponerinas. Los resultados sugieren que la diversidad es más alta de lo que se creía, tanto por que se encontraron nuevos registros durante la colecta de nuevo material, como porque nuestros análisis sugieren la presencia de especies crípticas. Adicionalmente, demostramos que en el PN Iguazú, dos ponerinas: Dinoponera australis y Pachycondyla striata son componentes dominantes en la comunidad de hormigas. Análisis de isótopos estables revelaron que la mayoría de las Ponerinas ocupan niveles tróficos altos, con excepción de algunas especies arborícolas del género Neoponera que dependerían de néctar u otros recursos vegetales.