Gelatinous Drop–Like Corneal Dystrophy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Trop2 and Its Overexpression in Cancers: Regulation and Clinical/ Therapeutic Implications
www.impactjournals.com/Genes&Cancer Genes & Cancer, Vol. 6 (3-4), March 2015 Trop2 and its overexpression in cancers: regulation and clinical/ therapeutic implications Anna Shvartsur1 and Benjamin Bonavida1 1 Department of Microbiology, Immunology and Molecular Genetics, Jonsson Comprehensive Cancer Center and David Geffen School of Medicine, University of California at Los Angeles, Los Angeles, CA, USA Correspondence to: Benjamin Bonavida, email: [email protected] Keywords: cancer, prognosis, resistance, signaling, Trop2 Received: September 29, 2014 Accepted: October 19, 2014 Published: October 19, 2014 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. ABSTRACT Trop2 is a transmembrane glycoprotein encoded by the Tacstd2 gene. It is an intracellular calcium signal transducer that is differentially expressed in many cancers. It signals cells for self-renewal, proliferation, invasion, and survival. It has stem cell-like qualities. Trop2 is expressed in many normal tissues, though in contrast, it is overexpressed in many cancers and the overexpression of Trop2 is of prognostic significance. Several ligands have been proposed that interact with Trop2. Trop2 signals the cells via different pathways and it is transcriptionally regulated by a complex network of several transcription factors. Trop2 expression in cancer cells has been correlated with drug resistance. Several strategies target Trop2 on cancer cells that include antibodies, antibody fusion proteins, chemical inhibitors, nanoparticles, etc. The in vitro studies and pre-clinical studies, using these various therapeutic treatments, have resulted in significant inhibition of tumor cell growth both in vitro and in vivo in mice. -

Original Article Mining Expression and Prognosis of TACSTD2 and SPP1 in Thyroid Carcinoma by Using Oncomine and Kaplan-Meier Plotter Databases
Int J Clin Exp Med 2019;12(9):11231-11240 www.ijcem.com /ISSN:1940-5901/IJCEM0094709 Original Article Mining expression and prognosis of TACSTD2 and SPP1 in thyroid carcinoma by using Oncomine and Kaplan-Meier plotter databases Qing Chang1, Mingda You1, Cui Li2 1Department of Head and Neck Surgery, Tangshan Worker Hospital of Hebei Province, Tangshan 063000, Hebei Province, PR China; 2Department of Neurosurgery, Yanghe People’s Hospital, Suqian 223800, Jiangsu Province, PR China Received April 1, 2019; Accepted June 11, 2019; Epub September 15, 2019; Published September 30, 2019 Abstract: Objective: To further investigate TACSTD2 and SPP1 gene mRNA expression in thyroid carcinoma and their association with patients’ prognosis and clinical pathological characteristics by data mining of the Oncomine and Kaplan-Meier plotter databases. Methods: TACSTD2 and SPP1 mRNA expression data from thyroid carcinoma were retrieved from the Oncomine database and further analyzed by comparing tumor tissue with corresponding normal tissue. The prognostic significance of TACSTD2 and SPP1 mRNA in thyroid carcinoma were evaluated by the online survival analysis tool “Kaplan-Meier Plotter” (KM plotter) database. Fifty-one patients with thyroid carcinoma who received surgery were retrospectively analyzed. The TACSTD2 and SPP1 protein expression in cancer tissue and nor- mal thyroid tissue were examined by immunohistochemical assay. The correlation between TACSTD2, SPP1 protein expression and patients’ clinicopathological characteristics were also evaluated. Results: Four analyses compared the TACSTD2 gene expression between thyroid cancer and normal tissue were included in the present work. The pooled results showed that TACSTD2 mRNA expression levels in thyroid carcinoma tissue was significantly higher than that of normal thyroid tissue (p=0.003). -

Systemic Surfaceome Profiling Identifies Target Antigens for Immune-Based Therapy in Subtypes of Advanced Prostate Cancer
Systemic surfaceome profiling identifies target antigens for immune-based therapy in subtypes of advanced prostate cancer John K. Leea,b,c, Nathanael J. Bangayand, Timothy Chaie, Bryan A. Smithf, Tiffany E. Parivaf, Sangwon Yung, Ajay Vashishth, Qingfu Zhangi,j, Jung Wook Parkf, Eva Coreyk, Jiaoti Huangi, Thomas G. Graeberc,d,l,m, James Wohlschlegelh, and Owen N. Wittec,d,f,n,o,1 aDivision of Hematology and Oncology, Department of Medicine, University of California, Los Angeles, CA 90095; bInstitute of Urologic Oncology, Department of Urology, University of California, Los Angeles, CA 90095; cJonsson Comprehensive Cancer Center, University of California, Los Angeles, CA 90095; dDepartment of Molecular and Medical Pharmacology, University of California, Los Angeles, CA 90095; eStanford University School of Medicine, Palo Alto, CA 94305; fDepartment of Microbiology, Immunology, and Medical Genetics, University of California, Los Angeles, CA 90095; gYale School of Medicine, New Haven, CT 06510; hDepartment of Biological Chemistry, University of California, Los Angeles, CA 90095; iDepartment of Pathology, Duke University School of Medicine, Durham, NC 27708; jDepartment of Pathology, China Medical University, 110001 Shenyang, People’s Republic of China; kDepartment of Urology, University of Washington School of Medicine, Seattle, WA 98195; lCrump Institute for Molecular Imaging, University of California, Los Angeles, CA 90095; mUCLA Metabolomics Center, University of California, Los Angeles, CA 900095; nParker Institute for Cancer Immunotherapy, -
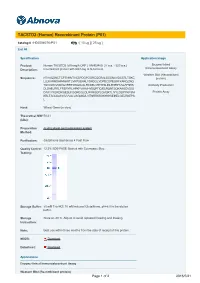
TACSTD2 (Human) Recombinant Protein (P01)
TACSTD2 (Human) Recombinant Protein (P01) Catalog # : H00004070-P01 規格 : [ 10 ug ] [ 25 ug ] List All Specification Application Image Product Human TACSTD2 full-length ORF ( AAH09409, 27 a.a. - 323 a.a.) Enzyme-linked Immunoabsorbent Assay Description: recombinant protein with GST-tag at N-terminal. Western Blot (Recombinant Sequence: HTAAQDNCTCPTNKMTVCSPDGPGGRCQCRALGSGMAVDCSTLTSKC protein) LLLKARMSAPKNARTLVRPSEHALVDNDGLYDPDCDPEGRFKARQCNQ TSVCWCVNSVGVRRTDKGDLSLRCDELVRTHHILIDLRHRPTAGAFNHS Antibody Production DLDAELRRLFRERYRLHPKFVAAVHYEQPTIQIELRQNTSQKAAGDVDIG DAAYYFERDIKGESLFQGRGGLDLRVRGEPLQVERTLIYYLDEIPPKFSM Protein Array KRLTAGLIAVIVVVVVALVAGMAVLVITNRRKSGKYKKVEIKELGELRKEPS L Host: Wheat Germ (in vitro) Theoretical MW 58.41 (kDa): Preparation in vitro wheat germ expression system Method: Purification: Glutathione Sepharose 4 Fast Flow Quality Control 12.5% SDS-PAGE Stained with Coomassie Blue. Testing: Storage Buffer: 50 mM Tris-HCI, 10 mM reduced Glutathione, pH=8.0 in the elution buffer. Storage Store at -80°C. Aliquot to avoid repeated freezing and thawing. Instruction: Note: Best use within three months from the date of receipt of this protein. MSDS: Download Datasheet: Download Applications Enzyme-linked Immunoabsorbent Assay Western Blot (Recombinant protein) Page 1 of 2 2016/5/21 Antibody Production Protein Array Gene Information Entrez GeneID: 4070 GeneBank BC009409 Accession#: Protein AAH09409 Accession#: Gene Name: TACSTD2 Gene Alias: EGP-1,GA733,GA733-1,M1S1,TROP2 Gene tumor-associated calcium signal transducer 2 Description: Omim ID: 137290, 204870 Gene Ontology: Hyperlink Gene Summary: This intronless gene encodes a carcinoma-associated antigen defined by the monoclonal antibody GA733. This antigen is a member of a family including at least two type I membrane proteins. It transduces an intracellular calcium signal and acts as a cell surface receptor. Mutations of this gene result in gelatinous drop-like corneal dystrophy, an autosomal recessive disorder characterized by severe corneal amyloidosis leading to blindness. -

Global Reactivation of Epigenetically Silenced Genes in Prostate Cancer
Published OnlineFirst August 10, 2010; DOI: 10.1158/1940-6207.CAPR-10-0039 Research Article Cancer Prevention See perspective p. 1053 Research Global Reactivation of Epigenetically Silenced Genes in Prostate Cancer Ilsiya Ibragimova1, Inmaculada Ibáñez de Cáceres1, Amanda M. Hoffman1, Anna Potapova1, Essel Dulaimi1, Tahseen Al-Saleem2, Gary R. Hudes3, Michael F. Ochs4, and Paul Cairns1,2 Abstract Transcriptional silencing associated with aberrant promoter hypermethylation is a common mechanism of inactivation of tumor suppressor genes in cancer cells. To globally profile the genes silenced by hypermethylation in prostate cancer, we screened a whole genome expression microarray for genes reactivated in the LNCaP, DU-145, PC-3, and MDA2b prostate tumor cell lines after treatment with the demethylating drug 5-aza-2-deoxycytidine and the histone deacetylation–inhibiting drug trichostatin A. A total of 2,997 genes showed at least 2-fold upregulation of expression after drug treatment in at least one prostate tumor cell line. For validation, we examined the first 45 genes, ranked by upregulation of expression, which had a typical CpG island and were known to be expressed in the normal cell counterpart. Two important findings were, first, that several genes known to be frequently hypermethylated in prostate cancer were apparent, and, second, that validation studies revealed eight novel genes hypermethylated in the prostate tumor cell lines, four of which were unmethylated in normal prostate cells and hypermethylated in primary prostate tumors (SLC15A3, 66%; KRT7, 54%; TACSTD2, 17%; GADD45b, 3%). Thus, we established the utility of our screen for genes hypermethylated in prostate cancer cells. One of the novel genes was TACSTD2/TROP2, a marker of human prostate basal cells with stem cell characteristics. -

Recent Advances in Trop 2 Targeted Therapy for Solid Tumors
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 2 December 2020 doi:10.20944/preprints202012.0062.v1 Minireview Recent advances in Trop 2 targeted therapy for solid tumors Shutan Liao1,*, Bing Wang1, Rong Zeng1, Haifeng Bao1, Xiaomin Chen1, Rakesh Dixit2 and Xiaoyan Xing1,* 1 Amador Bioscience Ltd, Hangzhou, China 2 Bionavigen LLC, Maryland, USA Abbreviated Title: Recent advances in Trop 2 targeted therapy *Address for correspondence: Xiaoyan Xing, MD, PhD 260 No.6 Street, Building 19, Suite 318, Qiantang New Area, Hangzhou, Zhejiang, China 310018 Email: [email protected] Shutan Liao, MD, PhD 260 No.6 Street, Building 19, Suite 318, Qiantang New Area, Hangzhou, Zhejiang, China 310018 Email: [email protected] Abstract Trophoblast cell-surface antigen 2 (Trop 2) is a transmembrane glycoprotein that is highly expressed in various cancer types with relatively low or no baseline expression in most of normal tissues. Its overexpression is associated with tumor growth and poor prognosis; Trop 2 is therefore, an ideal therapeutic target for epithelial cancers. Several Trop 2 targeted therapeutics have recently been developed for the treatment of cancers, such as anti-Trop 2 antibodies and antibody-drug conjugates (ADCs), as well as Trop 2-specific cell therapy. In particular, the safety and clinical benefit of Trop 2-based ADCs have been demonstrated in clinical trials across multiple tumor types, including those with limited treatment options, such as triple-negative breast cancer, platinum-resistant urothelial cancer, and heavily pretreated non-small cell lung cancer. In this review, we elaborate on recent advances in Trop 2 targeted modalities and provide an overview of novel insights for future developments in this field. -

Cell–Cell Communication at the Embryo Implantation Site of Mouse Uterus Revealed by Single-Cell Analysis
International Journal of Molecular Sciences Article Cell–Cell Communication at the Embryo Implantation Site of Mouse Uterus Revealed by Single-Cell Analysis Yi Yang 1, Jia-Peng He 2 and Ji-Long Liu 2,* 1 College of Veterinary Medicine, Gansu Agricultural University, Lanzhou 730070, China; [email protected] 2 Guangdong Laboratory for Lingnan Modern Agriculture, College of Veterinary Medicine, South China Agricultural University, Guangzhou 510642, China; [email protected] * Correspondence: [email protected] Abstract: As a crucial step for human reproduction, embryo implantation is a low-efficiency process. Despite rapid advances in recent years, the molecular mechanism underlying embryo implantation remains poorly understood. Here, we used the mouse as an animal model and generated a single- cell transcriptomic atlas of embryo implantation sites. By analyzing inter-implantation sites of the uterus as control, we were able to identify global gene expression changes associated with embryo implantation in each cell type. Additionally, we predicted signaling interactions between uterine luminal epithelial cells and mural trophectoderm of blastocysts, which represent the key mechanism of embryo implantation. We also predicted signaling interactions between uterine epithelial-stromal crosstalk at implantation sites, which are crucial for post-implantation development. Our data provide a valuable resource for deciphering the molecular mechanism underlying embryo implantation. Keywords: embryo implantation; mouse; single-cell RNA-seq; transcriptional changes Citation: Yang, Y.; He, J.-P.; Liu, J.-L. Cell–Cell Communication at the Embryo Implantation Site of Mouse Uterus Revealed by Single-Cell 1. Introduction Analysis. Int. J. Mol. Sci. 2021, 22, Embryo implantation is a crucial step for human reproduction. -

Research2007herschkowitzetvolume Al
Open Access Research2007HerschkowitzetVolume al. 8, Issue 5, Article R76 Identification of conserved gene expression features between comment murine mammary carcinoma models and human breast tumors Jason I Herschkowitz¤*†, Karl Simin¤‡, Victor J Weigman§, Igor Mikaelian¶, Jerry Usary*¥, Zhiyuan Hu*¥, Karen E Rasmussen*¥, Laundette P Jones#, Shahin Assefnia#, Subhashini Chandrasekharan¥, Michael G Backlund†, Yuzhi Yin#, Andrey I Khramtsov**, Roy Bastein††, John Quackenbush††, Robert I Glazer#, Powel H Brown‡‡, Jeffrey E Green§§, Levy Kopelovich, reviews Priscilla A Furth#, Juan P Palazzo, Olufunmilayo I Olopade, Philip S Bernard††, Gary A Churchill¶, Terry Van Dyke*¥ and Charles M Perou*¥ Addresses: *Lineberger Comprehensive Cancer Center. †Curriculum in Genetics and Molecular Biology, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA. ‡Department of Cancer Biology, University of Massachusetts Medical School, Worcester, MA 01605, USA. reports §Department of Biology and Program in Bioinformatics and Computational Biology, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA. ¶The Jackson Laboratory, Bar Harbor, ME 04609, USA. ¥Department of Genetics, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA. #Department of Oncology, Lombardi Comprehensive Cancer Center, Georgetown University, Washington, DC 20057, USA. **Department of Pathology, University of Chicago, Chicago, IL 60637, USA. ††Department of Pathology, University of Utah School of Medicine, Salt Lake City, UT 84132, USA. ‡‡Baylor College of Medicine, Houston, TX 77030, USA. §§Transgenic Oncogenesis Group, Laboratory of Cancer Biology and Genetics. Chemoprevention Agent Development Research Group, National Cancer Institute, Bethesda, MD 20892, USA. Department of Pathology, Thomas Jefferson University, Philadelphia, PA 19107, USA. Section of Hematology/Oncology, Department of Medicine, Committees on Genetics and Cancer Biology, University of Chicago, Chicago, IL 60637, USA. -

In TACSTD2 Among Iranian GDLD Patients
Four Mutations (Three Novel, One Founder) in TACSTD2 among Iranian GDLD Patients Afagh Alavi,1,2 Elahe Elahi,1,2,3 Mehdi Hosseini Tehrani,4 Fahimeh Asadi Amoli,4 Mohammad-Ali Javadi,5 Nasrin Rafati,5 Mohsen Chiani,6 Setareh Sadat Banihosseini,1,7 Behnaz Bayat,1 Reza Kalhor,1,8 and Seyed S. H. Amini 9 PURPOSE. To perform a mutation screening of TACSTD2 in 13 founder mutation in this population. Based on positions of Iranian Gelatinous Drop-like Corneal Dystrophy (GDLD) pedi- known mutations in TACSTD2, significance of the thyroglobu- grees. To assess genotype–phenotype correlations. To deter- lin domain of the TACSTD2 protein in the pathogenesis of mine intragenic SNP haplotypes associated with the mutations, GDLD is suggested. (Invest Ophthalmol Vis Sci. 2007;48: so as to gain information on their origin. 4490–4497) DOI:10.1167/iovs.07-0264 METHODS. The coding region of TACSTD2 was sequenced in the probands of 13 unrelated Iranian GDLD pedigrees. Variations were assessed in other available affected and unaffected family elatinous drop-like corneal dystrophy (GDLD, corneal fa- members and in unrelated normal control subjects by restric- Gmilial subepithelial amyloidosis; OMIM 204870; Online tion fragment length polymorphism (RFLP). The variations Mendelian Inheritance in Man; http://www.ncbi.nlm.nih.gov/ were classified as being associated with disease if they segre- Omim/ provided in the public domain by the National Center gated with the disease phenotype in the families, were not for Biotechnology Information, Bethesda, MD) is a rare inher- observed in 100 control individuals, disrupted protein expres- ited disease first described by Nakaizumi in 1914.1 It is char- sion, or affected conserved positions in the coded protein. -

Genetic Suppressor Element 1 (GSE1) Promotes the Oncogenic and Recurrent Phenotypes of Castration-Resistant Prostate Cancer by T
cancers Article Genetic Suppressor Element 1 (GSE1) Promotes the Oncogenic and Recurrent Phenotypes of Castration-Resistant Prostate Cancer by Targeting Tumor-Associated Calcium Signal Transducer 2 (TACSTD2) Oluwaseun Adebayo Bamodu 1,2,3 , Yuan-Hung Wang 2,4, Chen-Hsun Ho 5,6, Su-Wei Hu 1,4,7, Chia-Da Lin 1,7, Kai-Yi Tzou 1,7,8, Wen-Ling Wu 1,7, Kuan-Chou Chen 1,4,7,8 and Chia-Chang Wu 1,7,8,* 1 Department of Urology, Shuang Ho Hospital, Taipei Medical University, New Taipei City 235, Taiwan; [email protected] (O.A.B.); [email protected] (S.-W.H.); [email protected] (C.-D.L.); [email protected] (K.-Y.T.); [email protected] (W.-L.W.); [email protected] (K.-C.C.) 2 Department of Medical Research, Shuang Ho Hospital, Taipei Medical University, New Taipei City 235, Taiwan; [email protected] 3 Department of Hematology and Oncology, Shuang Ho Hospital, Taipei Medical University, New Taipei City 235, Taiwan 4 Graduate Institute of Clinical Medicine, College of Medicine, Taipei Medical University, Taipei City 110, Taiwan 5 Department of Surgery, Division of Urology, Shin Kong Wu Ho-Su Memorial Hospital, Citation: Bamodu, O.A.; Wang, Y.-H.; Taipei City 111, Taiwan; [email protected] Ho, C.-H.; Hu, S.-W.; Lin, C.-D.; Tzou, 6 School of Medicine, College of Medicine, Fu-Jen Catholic University, New Taipei City 242, Taiwan K.-Y.; Wu, W.-L.; Chen, K.-C.; Wu, 7 TMU Research Center of Urology and Kidney, Taipei Medical University, Taipei City 110, Taiwan 8 C.-C. -

DNA Methylation of Tumor Associated Calcium Signal
Katzendorn et al. BMC Cancer (2021) 21:444 https://doi.org/10.1186/s12885-021-08172-1 RESEARCH Open Access DNA methylation of tumor associated calcium signal transducer 2 (TACSTD2) loci shows association with clinically aggressive renal cell cancers Olga Katzendorn1†, Inga Peters1*†, Natalia Dubrowinskaja1, Hossein Tezval1, Pouriya Faraj Tabrizi1, Christoph A. von Klot1, Jörg Hennenlotter2, Marcel Lafos3, Markus A. Kuczyk1 and Jürgen Serth1 Abstract Background: DNA methylation is frequently observed in the development and progression of many human tumors as well as renal cell cancer (RCC). Tumor Associated Calcium Signal Transducer 2 (TACSTD2) participates in cell cycle progression through MAPK signalling pathway activation. Moreover, tumor-specific hypermethylation and association with aggressive cancer characteristics has been found for lung adenocarcinoma, hepatocellular carcinoma and cholangiocarcinoma. Whether TACSTD2 is tumor specifically hypermethylated in RCC or shows association of methylation with adverse clinicopathological parameters and survival of patients has not been investigated at yet. Methods: Quantitative methylation-specific PCR (qMSP) analysis of a locus in the intron 1 region of TACSTD2 gene was carried out in a cross-sectional study of 127 paired RCC and normal samples. In silico analysis of TACSTD2 methylation in the TCGA Kidney Renal Clear Cell Carcinoma (KIRC) dataset of 280 patients served as validation cohort. Statistical analyses were carried out using the two-sided paired t-test for matched tumor and normal sample comparisons, logistic regression for subgroup comparisons, Cox regression for analysis of recurrence free survival (RFS) and Pearson correlation analysis for correlation of TACSTD2 methylation and TACSTD2 mRNA in KIRC data. Results: Higher methylation levels in RCC were significantly associated with advanced disease (p < 0.001), high tumor stage (p = 0.003), tumor differentiation (p = 0.033) and presence of lymph node (p = 0.021) or distant metastases (p = 0.008). -

Identification and Characterization of a Novel TACSTD2 Mutation in Gelatinous Drop-Like Corneal Dystrophy
Molecular Vision 2010; 16:729-739 <http://www.molvis.org/molvis/v16/a83> © 2010 Molecular Vision Received 12 March 2010 | Accepted 17 April 2010 | Published 28 April 2010 Identification and characterization of a novel TACSTD2 mutation in gelatinous drop-like corneal dystrophy Preeti Paliwal,1 Jaya Gupta,2 Radhika Tandon,2 Namrata Sharma,2 Jeewan S. Titiyal,2 Seema Kashyap,2 Seema Sen,2 Punit Kaur,3 Divya Dube,3 Arundhati Sharma,1 Rasik B. Vajpayee2,4 1Laboratory of Cyto-Molecular Genetics, Department of Anatomy, All India Institute of Medical Sciences, New Delhi, India; 2Dr. Rajendra Prasad Centre for Ophthalmic Sciences, All India Institute of Medical Sciences, New Delhi, India; 3Department of Biophysics, All India Institute of Medical Sciences, New Delhi, India; 4Centre for Eye Research Australia, University of Melbourne, Australia Purpose: To study the clinical, histological, in vivo confocal microscopic, and molecular profile in a family with gelatinous drop-like corneal dystrophy (GDLD) from north India. Methods: Two siblings from a consanguineous family presented with clinical features analogous to GDLD. Detailed clinical evaluations were performed for all the available affected and unaffected members of this family. In vivo confocal microscopy and histology was done wherever necessary. DNA isolated from peripheral blood samples was subjected to polymerase chain reaction (PCR) followed by direct sequencing to detect mutations in the tumor-associated calcium signal transducer 2 (TACSTD2) gene. Protein modeling studies were done to asses the effect of the mutation on the protein structure. Results: The diagnosis of GDLD was established in the patient and the affected sibling on slit-lamp examinations, which revealed mulberry-like opacities in the subepithelium and anterior stroma that were confirmed on histopathology.