Influence of Environmental Features at Multiple Scales and Spatial Structure on Stream Fish Communities in a Tropical Agricultural Region
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DNA Barcode of Parodontidae Species from the La Plata River Basin - Applying New Data to Clarify Taxonomic Problems
Neotropical Ichthyology, 11(3):497-506, 2013 Copyright © 2013 Sociedade Brasileira de Ictiologia DNA barcode of Parodontidae species from the La Plata river basin - applying new data to clarify taxonomic problems Elisangela Bellafronte1, Tatiane Casagrande Mariguela2, Luiz Henrique Garcia Pereira2, Claudio Oliveira2 and Orlando Moreira-Filho1 In the past years, DNA barcoding has emerged as a quick, accurate and efficient tool to identify species. Considering the difficulty in identifying some Parodontidae species from the La Plata basin and the absence of molecular data for the group, we aimed to test the effectiveness of DNA barcoding and discuss the importance of using different approaches to solve taxonomic problems. Eight species were analyzed with partial sequences of Cytochrome c oxidase I. The mean intraspecific K2P genetic distance was 0.04% compared to 4.2% for mean interspecific K2P genetic distance. The analyses of distance showed two pairs of species with K2P genetic divergence lower than 2%, but enough to separate these species. Apareiodon sp. and A. ibitiensis, considered as the same species by some authors, showed 4.2% genetic divergence, reinforcing their are different species. Samples of A. affinis from the Uruguay and Paraguay rivers presented 0.3% genetic divergence, indicating a close relationship between them. However, these samples diverged 6.1% from the samples of the upper Paraná River, indicating that the latter represents a potentially new species. The results showed the effectiveness of the DNA barcoding method in identifying the analyzed species, which, together with the morphological and cytogenetic available data, help species identification. Nos últimos anos o DNA barcoding surgiu como uma ferramenta rápida, precisa e eficiente para identificar espécies. -

Sem Título-7
ECOLOGY OF A STREAM INHABITED BY Aspidoras fuscoguttatus 363 ECOLOGY OF A STREAM FROM UPPER PARANÁ RIVER BASIN INHABITED BY Aspidoras fuscoguttatus NIJSSEN & ISBRÜCKER, 1976 (SILURIFORMES, CALLICHTHYIDAE) ARAUJO, R. B. de1 and GARUTTI, V. 1, 2 1Centro de Aqüicultura da UNESP (CAUNESP), Jaboticabal, SP, Brazil 2Depto. de Zoologia e Botânica, IBILCE/UNESP, São José do Rio Preto, SP, Brazil Correspondence to: Renato Braz de Araujo, Rua Joana Anderi Chalela, 335, CEP 15030-650, São José do Rio Preto, SP, Brazil, e-mail: [email protected] Received January 14, 2002 – Accepted May 16, 2002 – Distributed August 31, 2003 (With 3 figures) ABSTRACT Most information on catfishes of the genus Aspidoras has focused species kept in aquaria, in non-natural conditions. Biotic and abiotic parameters of environment inhabited by Aspidoras fuscoguttatus, associated ichthyofauna, as well as strategies involved in the successful occupation of different microhabitats in a first order stream, located in São José do Rio Preto, São Paulo State, were investigated. Data collection was monthly performed at three segments (headwaters, middle course, and mouth) from August 1999 to July 2000. The greatest values of water temperature, conductivity, turbidity, alkalinity, width, depth, and current velocity were obtained in the rainy season. Dissolved oxygen levels showed significant differences among three segments in all sampling months, except for September. Substrate composition was predominantly sandy, followed by silt and clay in middle course and mouth in rainy as much as in dry seasons. In headwaters a large increase of the sandy fraction occurred in the rainy season. In the marginal and aquatic vegetation 30 species of macrophytes and 5 of macroalgae were identified. -

Zootaxa, Apareiodon Agmatos, a New Species from The
Zootaxa 1925: 31–38 (2008) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA Copyright © 2008 · Magnolia Press ISSN 1175-5334 (online edition) Apareiodon agmatos, a new species from the upper Mazaruni river, Guyana (Teleostei: Characiformes: Parodontidae) DONALD C. TAPHORN B.1, HERNÁN LÓPEZ-FERNÁNDEZ 2 & CALVIN R. BERNARD3 1Museo de Ciencias Naturales de Guanare, BioCentro, UNELLEZ, Mesa de Cavacas, Guanare 3310, Estado Portuguesa, Venezuela. E-mail: [email protected] 2Department of Natural History, Royal Ontario Museum, 100 Queen’s Park, Toronto, Ontario M5S 2C6, Canada. E-mail: [email protected] 3Center for the Study of Biodiversity, University of Guyana, Georgetown, Guyana. E-mail: [email protected] Abstract Apareiodon agmatos, new species, is described from the upper Mazaruni River and its tributaries, Essequibo Basin, in western Guyana. The new species is distinguishable from all other species of Parodontidae by having an incomplete lat- eral line. The scales of A. agmatos are more numerous than in any previously described parodontid. It has five incisor- like pedunculate teeth on the premaxilla aligned in a straight row, each with a large central spatulate cusp bordered on each side by a minute lateral cusp. The maxilla has two or infrequently three incisors. It shares an unusual pigmentation pattern of one dorsomedial and four lateral black stripes with A. gransabana, which was described from the neighboring upper Río Caroní drainage, Orinoco Basin. Apareiodon agmatos also shares with A. gransabana and Parodon guyanen- sis a higher number (5 versus 4) of teeth than other members of the genus in the premaxillary. Apareiodon agmatos and A. -

Siluriformes: Callichthyidae) in the Subterranean Domain of Northern and Northeastern Brazil
13 4 297 Tencatt et al NOTES ON GEOGRAPHIC DISTRIBUTION Check List 13 (4): 297–303 https://doi.org/10.15560/13.4.297 First report of armored catfishes Callichthyinae Bonaparte, 1838 (Siluriformes: Callichthyidae) in the subterranean domain of northern and northeastern Brazil Luiz Fernando Caserta Tencatt,1 Bruno Ferreira dos Santos,2 Maria Elina Bichuette3 1 Universidade Estadual de Maringá, Coleção Ictiológica do Núcleo de Pesquisas em Limnologia, Ictiologia e Aquicultura e Programa de Pós- Graduação em Ecologia de Ambientes Aquáticos Continentais, Av. Colombo, 5790, 87020-900 Maringá, Paraná, Brazil. 2 Universidade Federal de Mato Grosso do Sul, Programa de Pós-Graduação em Biologia Animal, Av. Costa e Silva, 79070-900 Campo Grande, Mato Grosso do Sul, Brazil. 3 Universidade Federal de São Carlos, Departamento de Ecologia e Biologia Evolutiva, Laboratório de Estudos Subterrâneos, Rodovia Washington Luis, km 235, 13565-905 São Carlos, São Paulo, Brazil. Corresponding author: Luiz Fernando Caserta Tencatt, [email protected] Abstract The first occurrence of the armored catfishes of the subfamily Callichthynae is reported in subterranean water bodies of northern and northeastern Brazil. The records include 3 species, each occurring in 1 of the 3 caves in the central and northeastern regions of Brazil: Callichthys callichthys from Casa do Caboclo cave, Sergipe state; Hoplosternum lit- torale from the Gruna da Lagoa do Meio, Bahia state; and Megalechis thoracata, from Casa de Pedra cave, Tocantins state. Keywords Camboatá, cave, hypogean habitat, karstic areas, Neotropical region. Academic editor: Bárbara Calegari | Received 2 March 2017 | Accepted 10 June 2017 | Published 14 August 2017 Citation: Tencatt LFC, Ferreira dos Santos B, Bichuette ME (2017) First report of armored catfishes Callichthyinae( Bonaparte, 1838) (Siluriformes: Callichthyidae) in the subterranean domain. -

Cytogenetic Studies in Some Apareiodon Species (Pisces, Parodontidae)
C 2000 The Japan Mendel Society Cytologia 65: 397-402,2000 Cytogenetic Studies in Some Apareiodon Species (Pisces, Parodontidae) Celia Maria de Jesus* and Orlando Moreira-Filho Departamento de Genetica e Evolucbo, Universidade Federal de Sdo Carlos Rodovia Washington Luis, km 235, Caixa Postal 676, CEP 13565-905, Sdo Carlos, SP, Brasil Accepted September 8, 2000 Summary Cytogenetic studies in 6 species of Apareiodon (Pisces, Parodontidae) from different Brazilian hydrographic basins showed a diploid number equal to 2n=54 chromosomes and the ab- sence of morphologically differentiated sex chromosomes. Although the diploid and the fundamental numbers had been constant, some differences were observed concerning the karyotypic structure of the species. A. ibitiensis, Apareiodon sp. A and Apareiodon sp. B presented 50M/SM+4ST chromo- somes, while A. piracicabae, A. vittatus and Apareiodon sp. C presented 52M/SM+2ST ones. Apareiodon piracicabae showed a polymorphism in relation to the number and position of the nucle- olar organizer regions (NORs), while A. vittatus showed a variation in the NORs size. The available data indicate that the Parodontidae family have been submitted to a chromosomal diversification dur- ing their species differentiation process, despite the maintenance of the same diploid number. Key words Apareiodon, Karyotypic evolution, NOR, C-band. The Characiformes fish are widely distributed in the neotropical region, showing a wide varia- tion in the diploid number. Two general trends in the chromosomal evolution can be observed in this order. In fact, some groups show a heterogeneous evolutionary with a diversity in the chromo- some number and the karyotypic structure. In contrast, a more homogenous pattern can be observed in other groups, leading to a relatively stable karyotypes. -

First Record of the Family Parodontidae (Characiformes) from the Paraíba Do Sul River Basin, Southeastern Brazil
13 6 1091 NOTES ON GEOGRAPHIC DISTRIBUTION Check List 13 (6): 1091–1095 https://doi.org/10.15560/13.6.1091 First record of the family Parodontidae (Characiformes) from the Paraíba do Sul river basin, southeastern Brazil Fernando L. K. Salgado,1, 2 Bianca de F. Terra,3 Geysa da S. Camilo,1 F. Gerson Araújo1 1 Universidade Federal Rural do Rio de Janeiro, Departamento de Biologia Animal, Laboratório de Ecologia de Peixes, BR 465, km 7, CEP 23890- 00, Seropédica, RJ, Brazil. 2 Universidade Federal do Rio de Janeiro, Departamento de Zoologia, Laboratório de Sistmática e Evolução de Peixes Teleósteos, Avenida Carlos Chagas Filho, Cidade Universitária, CEP 21941-902, Rio de Janeiro, RJ, Brazil. 3 Universidade Estadual Vale do Acaraú, Centro de Ciências Agrárias e Biológicas, Campus da Betânia, CEP 62040-370, Sobral, CE, Brazil. Corresponding author: F. Gerson Araújo, [email protected] Abstract The first records of 2 species of Parodontidae (Apareiodon piracicabae and A. itapicuruensis) are reported in the Paraíba do Sul river basin. In total, 101 individuals of A. piracicabae and 3 individuals of A. itapicuruensis were collected in the Paraíba do Sul middle reaches. A description and diagnosis of both species based on morpho-meristic characters were provided. These fishes have been used as forage for larger fish species as well as bait for sport fishing, which may have facilitated their introduction in the Paraíba do Sul River from fish culture farms in the region. Key words Apareiodon; geographic distribution; occurrence; Paraíba do Sul River. Academic editor: Gabriela Echevarría | Received 5 August 2017 | Accepted 30 October 2017 | Published 22 December 2017 Citation: Salgado FLK, Terra BF, Camilo GS, Araújo FG (2017) First record of the family Parodontidae (Characiformes) from the Paraíba do Sul river basin, southeastern Brazil. -

Aspidoras Mephisto, New Species: the First Troglobitic Callichthyidae (Teleostei: Siluriformes) from South America
RESEARCH ARTICLE Aspidoras mephisto, new species: The first troglobitic Callichthyidae (Teleostei: Siluriformes) from South America Luiz Fernando Caserta Tencatt1*, Maria Elina Bichuette2 1 Departamento de Biologia, NuÂcleo de Pesquisas em Limnologia, Ictiologia e Aquicultura, Programa de PoÂs-GraduacËão em Ecologia de Ambientes AquaÂticos Continentais, Universidade Estadual de MaringaÂ, MaringaÂ, ParanaÂ, Brazil, 2 Departamento de Ecologia e Biologia Evolutiva, LaboratoÂrio de Estudos SubterraÃneos, Universidade Federal de São Carlos, São Carlos, São Paulo, Brazil a1111111111 a1111111111 * [email protected] a1111111111 a1111111111 a1111111111 Abstract Aspidoras mephisto n. sp. is described from the AneÂsio-Russão cave system, upper Tocan- tins River basin, GoiaÂs, Brazil. The species can be readily distinguished from its congeners OPEN ACCESS by troglomorphic features and also by presenting the following combination of features: Citation: Tencatt LFC, Bichuette ME (2017) infraorbital 1 generally with well-developed ventral laminar; or moderately developed; Aspidoras mephisto, new species: The first poorly-developed serrations on posterior margin of pectoral spine; nuchal plate not exter- troglobitic Callichthyidae (Teleostei: Siluriformes) nally visible; dorsal fin, even in conspicuously colored specimens, with only dark brown or from South America. PLoS ONE 12(3): e0171309. black chromatophores concentrated on rays, forming spots in some specimens; mem- doi:10.1371/journal.pone.0171309 branes hyaline; or sparse dark brown or black chromatophores on membranes, not forming Editor: Riccardo Castiglia, Universita degli Studi di any conspicuous pattern; and inner laminar expansion of infraorbital 1 moderately devel- Roma La Sapienza, ITALY oped. Information about its habitat, ecology, behaviour and conservation status are provided Received: October 11, 2016 and also a brief description of the juvenile stage. -

Relationships Between Environmental Conditions and Fish Assemblages in Tropical Savanna Headwater Streams
www.nature.com/scientificreports OPEN Relationships Between Environmental Conditions And Fish Assemblages In Tropical Savanna Headwater Streams Thiago Bernardi Vieira 1,2* & Francisco Leonardo Tejerina-Garro 3,4 Riparian vegetation plays an important role in providing energy to small watercourses and maintaining ecological processes through organic matter input and together with hydrological and geomorphological watercourse characteristics infuence on fsh assemblages. The goal of this paper was partitioning and quantifying the infuence of riparian zone (type of riverbank substrate, bank slope, type of riparian vegetation cover and percentage of riparian vegetation cover on the main channel), physical habitat (stream channel width and depth, type of substrate and aquatic habitat in channel, water velocity and organic matter), water quality (turbidity, temperature, conductivity, pH, dissolved oxygen and chlorophyll concentration) and spatial variables (linear distances between sampled points) on fsh assemblages (richness and abundance per species) in headwater streams of the Upper Paraná River basin, Central Brazil. For this purpose, it was performed a variation partitioning analysis between riparian, physical habitat, water and spatial variables sets and a Redundancy Analysis to quantify the infuence of variables on the fsh assemblages. Only the physical habitat and water quality variables infuenced the fsh assemblages (richness and abundance per species). Freshwater fsh assemblages are structured by variables related to both water quality and riparian vegetation1–6. In this sense, warmer waters exhibit higher fsh abundance and biomass while highly oxygenated waters may lead to greater species diversity7–9. Riparian vegetation is a transitional semiterrestrial system10 that provides energy in watercourses through the input of organic matter2. Leaves deposited on the watercourse bed contribute indirectly to fsh food because they act as a substrate for numerous microorganisms11 and insects12,13. -
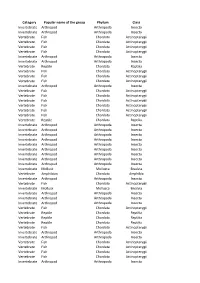
Category Popular Name of the Group Phylum Class Invertebrate
Category Popular name of the group Phylum Class Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Vertebrate Reptile Chordata Reptilia Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Invertebrate Arthropod Arthropoda Insecta Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Reptile Chordata Reptilia Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Mollusk Mollusca Bivalvia Vertebrate Amphibian Chordata Amphibia Invertebrate Arthropod Arthropoda Insecta Vertebrate Fish Chordata Actinopterygii Invertebrate Mollusk Mollusca Bivalvia Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Vertebrate -

Redalyc.Dieta Do Cascudo Aspidoras Fuscoguttatus (Ostariophysi
Biota Neotropica ISSN: 1676-0611 [email protected] Instituto Virtual da Biodiversidade Brasil Casatti, Lilian; Veronezi Júnior, José Luis; de Paula Ferreira, Cristiane Dieta do cascudo Aspidoras fuscoguttatus (Ostariophysi, Callichthyidae) em riachos com diferentes características limnológicas e estruturais Biota Neotropica, vol. 9, núm. 1, enero-marzo, 2009, pp. 113-121 Instituto Virtual da Biodiversidade Campinas, Brasil Disponível em: http://www.redalyc.org/articulo.oa?id=199115787019 Como citar este artigo Número completo Sistema de Informação Científica Mais artigos Rede de Revistas Científicas da América Latina, Caribe , Espanha e Portugal Home da revista no Redalyc Projeto acadêmico sem fins lucrativos desenvolvido no âmbito da iniciativa Acesso Aberto Biota Neotrop., vol. 9, no. 1, Jan./Mar. 2009 Dieta do cascudo Aspidoras fuscoguttatus (Ostariophysi, Callichthyidae) em riachos com diferentes características limnológicas e estruturais Lilian Casatti1,2, José Luis Veronezi Júnior1 & Cristiane de Paula Ferreira1 1Laboratório de Ictiologia, Departamento de Zoologia e Botânica, IBILCE, Universidade Estadual Paulista – UNESP, Rua Cristóvão Colombo, 2265, Jardim Nazareth, CEP 15054-000, São José do Rio Preto, SP, Brasil, e-mail: [email protected], [email protected] 2Autor para correspondência: Lilian Casatti, e-mail: [email protected] CASATTI, L., VERONEZI JÚNIOR, J.L. & FERREIRA, C.P. Diet of the armored catfish Aspidoras fuscoguttatus (Ostariophysi, Callichthyidae) in streams with different limnological and structural features. Biota Neotrop. 9(1): http://www.biotaneotropica.org.br/v9n1/en/abstract?article+bn02109012009. Abstract: In the present study Aspidoras fuscoguttatus Nijssen & Isbrücker, 1976 diet was investigated based on specimens from 18 streams of São Paulo State northwestern region, upper Rio Paraná system. Stomach contents of 246 specimens were analyzed, with the registration of 26 types of feeding items showing high predominance of the autochthonous ones. -
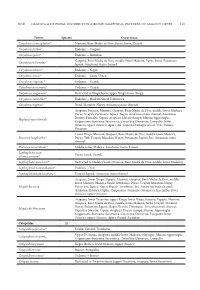
DAGOSTA & DE PINNA: DISTRIBUTION & BIOGEOGRAPHICAL PATTERNS of AMAZON FISHES Taxon Species Occurrence Corydoras Stenocep
2019 DAGOSTA & DE PINNA: DISTRIBUTION & BIOGEOGRAPHICAL PATTERNS OF AMAZON FISHES 113 Taxon Species Occurrence Corydoras stenocephalus* Mamoré, Beni-Madre de Dios, Purus, Juruá, Ucayali Corydoras sterbai* Endemic – Guaporé Corydoras sychri* Endemic – Marañon Guaporé, Beni-Madre de Dios, middle-lower Madeira, Purus, Juruá, Putumayo, Corydoras trilineatus* Japurá, Amazonas main channel Corydoras tukano* Endemic – Negro Corydoras urucu* Endemic – Coari-Urucu Corydoras virginiae* Endemic – Ucayali Corydoras weitzmani* Endemic – Ucayali Corydoras xinguensis* Restricted to Xingu basin (upper Xingu, lower Xingu) Corydoras zawadzkii* Endemic – Madeira Shield Tributaries Corydoras zygatus* Juruá, Marañon-Nanay, Amazonas main channel Araguaia, Juruena, Mamoré, Guaporé, Beni-Madre de Dios, middle-lower Madeira, Purus, Ucayali, Putumayo, Japurá, Negro, Amazonas main channel, Amazonas Estuary, Parnaíba, Capim, Araguari-Macari-Amapá, Maroni-Approuague, Hoplosternum littorale Coppename-Suriname-Saramacca, Corentyne-Demerara, Essequibo, lower Orinoco, upper Orinoco, Apure, Atl. Coastal Drainages of Col. Ven., Paraná- Paraguay Lower Xingu, Mamoré, Guaporé, Beni-Madre de Dios, middle-lower Madeira, Dianema longibarbis* Purus, Tefé, Ucayali, Marañon-Nanay, Putumayo, Japurá, Jari, Amazonas main channel Dianema urostriatum* Middle-lower Madeira, Amazonas main channel Lepthoplosternum Purus, Juruá, Ucayali altamazonicum* Lepthoplosternum beni* Restricted to Madeira basin (Mamoré, Beni-Madre de Dios, middle-lower Madeira) Lepthoplosternum stellatum* Endemic -

Long Term Evolutionary Responses to Whole Genome Duplication
Unicentre CH-1015 Lausanne http://serval.unil.ch Year : 2016 Long term evolutionary responses to whole genome duplication Sacha Laurent Sacha Laurent, 2016, Long term evolutionary responses to whole genome duplication Originally published at : Thesis, University of Lausanne Posted at the University of Lausanne Open Archive http://serval.unil.ch Document URN : urn:nbn:ch:serval-BIB_8E5536BAC6042 Droits d’auteur L'Université de Lausanne attire expressément l'attention des utilisateurs sur le fait que tous les documents publiés dans l'Archive SERVAL sont protégés par le droit d'auteur, conformément à la loi fédérale sur le droit d'auteur et les droits voisins (LDA). A ce titre, il est indispensable d'obtenir le consentement préalable de l'auteur et/ou de l’éditeur avant toute utilisation d'une oeuvre ou d'une partie d'une oeuvre ne relevant pas d'une utilisation à des fins personnelles au sens de la LDA (art. 19, al. 1 lettre a). A défaut, tout contrevenant s'expose aux sanctions prévues par cette loi. Nous déclinons toute responsabilité en la matière. Copyright The University of Lausanne expressly draws the attention of users to the fact that all documents published in the SERVAL Archive are protected by copyright in accordance with federal law on copyright and similar rights (LDA). Accordingly it is indispensable to obtain prior consent from the author and/or publisher before any use of a work or part of a work for purposes other than personal use within the meaning of LDA (art. 19, para. 1 letter a). Failure to do so will expose offenders to the sanctions laid down by this law.