Aspergillus Flavus NRRL 3251 Growth, Oxidative Status, And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ED611743.Pdf
ISSN 1849-7209 FACULTY OF EDUCATION JOSIP JURAJ STROSSMAYER UNIVERSITY OF OSIJEK CROATIAN ACADEMY OF SCIENCES AND ARTS THE CENTER FOR SCIENTIFIC WORK IN VINKOVCI EDITORS VESNICA MLINAREVIĆ, MAJA BRUST NEMET, JASMINA HUSANOVIĆ PEHAR INTERCULTURAL EDUCATION OBRAZOVANJE ZA INTERKULTURALIZAM CONFERENCE PROCEEDINGS 4th International Scientific and Professional Conference INTERCULTURAL EDUCATION Osijek, September 17th – 18th 2020 OSIJEK, 2021 Title Intercultural Education Obrazovanje za interkulturalizam Publisher Faculty of Education, Josip Juraj Strossmayer University of Osijek, Republic of Croatia Croatian Academy of Sciences and Arts, the Center for Scientific Work in Vinkovci, Republic of Croatia For the publisher Damir Matanović, PhD, Professor Editors Vesnica Mlinarević, PhD, Professor (Republic of Croatia) Maja Brust Nemet, PhD, Assistant Professor (Republic of Croatia) Jasmina Husanović Pehar, PhD, Associate Professor (Bosnia and Herzegovina) Technical editor Maja Brust Nemet, PhD, Assistant Professor Proofreading Ivana Moritz, PhD, Assistant Professor Cover design Goran Kujundžić, PhD, Associate Professor in Art Prepress and printing Zebra, Vinkovci January, 2021 ISSN 1849-7209 All rights reserved. No part of this book may be reproduced or utilized in any form of by any means, without permission in writing form from the publisher. Edition 150 copies Reviewers of Conference Proceedings Damir Arsenijević, PhD, Professor (Bosnia and Herzegovina) Smiljana Zrilić, PhD, Professor (Republic of Croatia) Reviewers of papers Josip Ivanović, -

Ferit-Brochure.Pdf
FACULTY OF ELECTRICAL ENGINEERING, COMPUTER SCIENCE AND INFORMATION TECHNOLOGY OSIJEK Dean’s foreword The Faculty of Electrical Engineering, Computer Science and Information Technology Osijek is a mod- ern faculty that has constantly been developing in all areas of its expertise. The educational process consists of three levels with study programmes being continually upgraded and harmonised with recent scientific discoveries and economy needs. Scientific research is essential for the development of the Faculty. The Faculty actively participates in numerous domestic and international consortia used by the Faculty’s established research groups for conducting successful scientific research. The Faculty’s research groups have participated in nu- merous domestic and European projects as either heads or associates. Cooperation with the economy, encouragement of entrepreneurship and transfer of knowledge and technologies are fundamental indicators of the Faculty’s development. The Faculty successfully cooperates with the most important companies in the fields of electrical engineering, computer engineering and information and communication technologies. Cooperation with the economy has been supported by professional and development projects and research studies. Furthermore, do- ing their practical work, bachelor and master theses, students have gradually been prepared for the job market and solving real engineering problems. Undoubtedly, powerful faculties and universities are imperatives for the society’s development. In- formation and communication technology, electrical and computer engineering providing addi- tional values for new products and services represent strategic priorities for the development of modern economies. Hence, the Faculty of Electrical Engineering, Computer Science and Information Technology Osijek continues to successfully fulfil its mission – provision of support for knowledge and technology-based economy. -

3. Osijek-Baranja County Basic Information
CONTENTS 1. FOREWORD .........................................................................................................................................................5 Published by 2. REPUBLIC OF CROATIA ........................................................................................................................................7 Osijek - Baranja County 2.1. Basic information..............................................................................................................................................7 For publisher 2.2. Administrative and political structure ........................................................................................................7 Krešimir Bubalo, County prefect 2.3. Geographical position ....................................................................................................................................8 2.4. Economy .............................................................................................................................................................8 Developed by Center for Entrepreneurship Osijek 3. OSIJEK-BARANJA COUNTY ..................................................................................................................................9 3.1. Basic information ............................................................................................................................................10 Project coordination 3.2. Geographical position ...................................................................................................................................10 -

Partner-Institutions Study - Opportunities and Short-Time School Practice Nr
Partner-Institutions Study - opportunities and short-time school practice Nr. Country Place Partner-Institution 1 Austria St. Jakob im Rosental Volksschule 2 Austria St. Jakob im Rosental NMS / Hauptschule 3 Austria St. Jakob im Rosental Höhere Lehranstalt für wirtschaftliche Berufe 4 Belgium Bastogne University College Namur-Liège-Luxembourg 5 Belgium Geel University College Thomas More Kempen 6 Belgium Kortrijk VIVES University College 7 Belgium Namur University College Albert Jacquard 8 Bosnia and Herzegovina Travnik University Vitez Travnik 9 Bulgaria Veliko Turnovo University of Veliko Turnovo / Faculty of Modern Languages 10 Bulgaria Veliko Turnovo University of Veliko Turnovo / Faculty of Mathematics and Informatics 11 Chile Santiago University Mayor 12 Chile Santiago Instituto Profesional Alemán Wilhelm von Humboldt 13 Chile Santiago Lehrerbildunginstitut 14 China Hangzhou Hangzhou Normal University 15 Croatia Osijek University of Osijek 16 Croatia Rijeka University of Rijeka 17 Croatia Zagreb University of Zagred 18 Czech Republic Brno Masaryk University / Department of German Language and Literature 19 Czech Republic Olomouc Palacký University Olomouc 20 Czech Republic Pilsen University of West Bohemia 21 Czech Republic Usti nad Labem Jan Evangelista Purkyne University in Usti nad Labem / Faculty of Education 22 Czech Republic Usti nad Labem Jan Evangelista Purkyne University in Usti nad Labem / Faculty of Philosophy 23 Denmark Haderslev University College South Denmark 24 Ecuador Quito Escuela 27 de Febrero 25 Estonia Tallinn -

ROP OBZ Engl 130906
OSIJEK-BARANJACOUNTY REGIONALOPERATING PROGRAM2006-2013 The 2006 to 2013 Regional Operational Program of the Osijek and Baranja County REGIONAL OPERATING PROGRAM 2006 - 2013 OSIJEK-BARANJA COUNTY The 2006 to 2013 Regional Operational Program of the Osijek and Baranja County IMPORTANT NOTICE All questions in relation to the contents of the ROP please send to the following address: Agency for development Europske avenije 11 Tel: 031/200 677 The County web site: www.obz.hr PROTECTION OF RIGHTS All the contents of this ROP is exclusively made for the use of the Osijek-Baranja County. Inženjerski biro d.d. Zagreb has elaborated this Regional Operational Program in compliance with the client Osijek-Baranja County's requirements, only for its specific implementation. All other person using information set out in this Program are doing so to their own responsibility and risk. This Program has been translated from Croatian to English language. In case of misinterpretation the Croatian version is valid. The 2006 to 2013 Regional Operational Program of the Osijek and Baranja County C O N T E N T S Preface..................................................................................................................................................... 1 Introduction ............................................................................................................................................. 3 I. BASIC ANALYSIS ............................................................................................................................ -
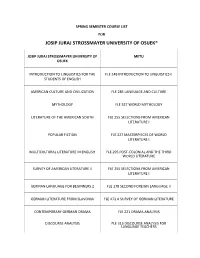
Josip Juraj Strossmayer University of Osijek*
SPRING SEMESTER COURSE LIST FOR JOSIP JURAJ STROSSMAYER UNIVERSITY OF OSIJEK* JOSIP JURAJ STROSSMAYER UNIVERSITY OF METU OSIJEK INTRODUCTION TO LINGUISTICS FOR THE FLE 248 INTRODUCTION TO LINGUISTICS II STUDENTS OF ENGLISH AMERICAN CULTURE AND CIVILIZATION FLE 285 LANGUAGE AND CULTURE MYTHOLOGY FLE 327 WORLD MYTHOLOGY LITERATURE OF THE AMERICAN SOUTH FLE 255 SELECTIONS FROM AMERICAN LITERATURE I POPULAR FICTION FLE 227 MASTERPIECES OF WORLD LITERATURE I MULTICULTURAL LITERATURE IN ENGLISH FLE 295 POST-COLONIAL AND THE THIRD WORLD LITERATURE SURVEY OF AMERICAN LITERATURE II FLE 255 SELECTIONS FROM AMERICAN LITERATURE I GERMAN LANGUAGE FOR BEGINNERS 2 FLE 178 SECOND FOREIGN LANGUAGE II GERMAN LITERATURE FROM SLAVONIA FLE 471 A SURVEY OF GERMAN LITERATURE CONTEMPORARY GERMAN DRAMA FLE 221 DRAMA ANALYSIS DISCOURSE ANALYSIS FLE 313 DISCOURSE ANALYSIS FOR LANGUAGE TEACHERS VENICE IN THE MIDDLE AGES FLE 120 HISTORY OF IDEAS I MEDIEVAL NOBILITY FLE 120 HISTORY OF IDEAS I EMPIRE AND PAPACY FLE 120 HISTORY OF IDEAS I RESEARCH METHODS I FLE 311 ADVANCED WRITING RESEARCH SKILLS / EDS 240 RESEARCH METHODS IN EDUCATION THE FUNDAMENTALS OF CRITICAL THINKING FLE 120 HISTORY OF IDEAS / FLE 251 CRITICAL READING AND WRITING WORD FORMATION IN CONTEMPORARY FLE 476 LEXICAL STRUCTURE AND WORD GERMAN FORMATION IN GERMAN THE MEDIEVAL PERIOD OF WORLD HISTORY FLE 120 HISTORY OF IDEAS I INTRODUCTION TO THE LINGUISTICS FOR FLE 472 INTRODUCTION TO GERMAN STUDENTS OF GERMAN LINGUISTICS SYNTAX OF THE COMPLEX SENTENCE IN FLE 472 INTRODUCTION TO GERMAN CONTEMPORARY GERMAN -

Energy Efficiency of Tram Transport in the City of Osijek
Energy Efficiency of Tram Transport in the City of Osijek Case Study Hrvoje Glavaš J. J. Strossmayer University of Osijek, Faculty of Electrical Engineering Kneza Trpimira bb, Osijek, Croatia [email protected] Milan Ivanović Panon – Think-tank for Strategic Studies Osijek, Croatia [email protected] Abstract – In the city of Osijek, tram transport started long before it was introduced in many European and world cities. This paper presents the ways to increase tram traffic energy efficiency in the city of Osijek. The tram transportation system as an urban way of traffic as well as plans for further development are described. Energy efficiency indicators are presented based on electricity consumption and the number of passengers in the past decade. The tram transport system is compared with other forms of public transport in the city of Osijek. An increase in energy efficiency in the sector of urban transport is proved based on the presented data. Keywords – Electricity Consumption; Energy Efficiency; Public Transport; Tram 1. INTRODUCTION in Pula, in 1935 in Opatija, and in Rijeka and Dubrovnik in the late 70ies). [1] The city of Osijek (around 115,000 inhabitants) is suitable for the organization of urban public trans- 1.2. PUBLIC TRANSPORTATION TODAY port; a narrower part of the city is situated along the Drava River within approximately 13 x 3 km. A long Public transport is performed by trams and buses tradition of tram transportation and public transpor- (a public company named GPP) and taxicabs (twenty tation in Osijek is described in the paper. The paper private companies with fifty cars, two largest ones are analyzes energy efficiency in public transport, espe- “Osijek taxi” and “Cameo”). -

Enhancing Academic Mobility in Croatia: Croatia's Entry Into Erasmus
Enhancing Academic Mobility in Croatia: Croatia’s Entry into Erasmus HANDBOOK Institute for the Development of Education Zagreb, 2008 Title of the handbook Translation and proof-reading Enhancing Academic Mobility in Croatia: Martina Kado Croatia’s Entry into Erasmus Mirna Furdek Nina Vranešević Publisher Institute for the Development of Education Printing and binding Preradovićeva 33/I, 10000 Zagreb, Croatia Bauer grupa www.iro.hr Graphic design Editor-in-Chief Netipični Ninoslav Šćukanec Zagreb, 2008. Executive Editor Thomas Farnell This project has been funded with support from the European Commission. This publication reflects the views only of the author, and the Commission cannot be held responsible for any use which may be made of the information contained therein. The publication “Enhancing Academic Mobility in Croatia: Croatia’s Entry into Erasmus” was published through the pro- ject “Enhancing Mobility of the Croatian Academic Community (MOBIL)”, which is financed by the European Commission through the Tempus programme (Grant Agreement number SCM C018B06-2006 HR). A C.I.P. catalogue record for this publication is available from the National and University Library in Zagreb under the code number 668716. The project is funded by A project coordinated by the Institute ISBN 978-953-95614-1-1 the European Commision for the Development of Education Table of contents Editor’s note Ninoslav Šćukanec, President, Institute for the Development of Education, Croatia ............................................. 07 Foreword Prof. Daniel Rukavina, M.D., D.Sc., Rector of the University of Rijeka, President of the Croatian Rector’s Conference, Croatia .............................................................................................................................. 10 Radovan Fuchs, Ph.D., State Secretary for Higher Education and International Cooperation, Ministry of Science, Education and Sports, Croatia ................................................................................................................. -

Josip Juraj Strossmayer University of Osijek Croatia
UNIVERSITY OF OSIJEK CROATIA The newest EU member state South-East Europe → Croatia → North-East Croatia → Osijek-Baranja County → OSIJEK THE CITY OF OSIJEK HISTORICAL OVERVIEW The history of Osijek dates back to Neolithic times. Osijek was first mentioned in 12th century as Eszek. Few municipalities: Lower Town, Tvrđa, and Upper Town were united in 1786, it resulted with large economic and cultural development, so during the early 19th century Osijek was the largest city in Croatia. Osijek was continuously growing in 19th and 20th century, so Osijek became a regional administrative and cultural centre. Osijek was heavily damaged during the Croatian War of Independence in 1990s. OSIJEK TODAY Administrative, economic and cultural centre of Eastern Croatia. Population (2011.) -108 048 City of squares (15). City of parks (17). University of Osijek (UNIOS) was founded on 31st May 1975 FACULTIES AND DEPARTMENTS: Faculty of Humanities and Social Sciences Faculty of Economics Faculty of Electrical Engineering Faculty of Philosophy Faculty of Civil Engineering Catholic Faculty of Theology Faculty of Medicine Faculty of Agriculture Faculty of Law Faculty of Food Technology Faculty of Mechanical Engineering Faculty of Education Academy of Arts Departments (Mathematics, Physics, Biology, Chemistry, Cultural Studies) STUDY PROGRAMMES FACULTY OF HUMANITIES AND SOCIAL SCIENCES GENERAL INFORMATION: Due to Osijek’s geographical location, the Faculty of Humanities and Social Sciences has been designated as an international research centre of humanities and social studies in this part of Europe. A wide variety of study programmes at the undergraduate, graduate and postgraduate levels, as well as programmes in the area of lifelong learning facilitate fostering of interdisciplinarity between the humanities and social sciences, but also within the fields themselves. -

1St International Students' GREEN Conference
First Circular Celebrating International Day for Biological Diversity, we are invited you to participate at the 1st International Students’ GREEN Conference which will be held 17-18 May 2018 in Osijek, Croatia It is our great pleasure to invite you to participate IMPORTANT DATES ORGANIZERS in the 1 st International Students’ GREEN April 16th 2018 – abstract submission • Josip Juraj Strossmayer University of Osijek Conference, which will take place in Osijek, Croatia April 24th 2018 – notification regarding acceptance of • Faculty ofa Agriculture in Osijek from May 17 th to 18 th 2018. abstract • Faculty of Civil Engineering Osijek May 10th 2018 – registration of participants • Faculty of Economics in Osijek The 1st International Students’ GREEN Conference June 18th 2018 – full paper submission • Faculty of Dental Medicine and Health Osijek (ISC GREEN 2018 ) is organised by the Josip Juraj *participation is free of charge but registration is required • Faculty of Electrical Engineering, Computer Science and Information Technology Osijek Strossmayer University of Osijek in collaboration • Faculty of Teacher Education in Osijek with the International Federation of Environmental CONTACT • Faculty of Humanities and Social Sciences Osijek Health and the network of higher education and Mirna Habuda-Stanić Faculty of Food Technology Osijek • Catholic Faculty of Theology in Đakovo research institutions. Franje Kuhača 20, 31000 Osijek, Croatia • Faculty of Medicine Osijek This conference aims at bringing together students tel.: +385 31 224 326 • Faculty of Law Osijek • Faculty of Food Technology Osijek of natural, biomedical, technical, biotechnical, faks: +385 31 207 115 e-mail: [email protected] • Mehanical Engineering Faculty in Slavonski Brod humanities and social sciences, as well as enabling • Academy of Arts in Osijek the exchange and broadening of students’ • Josip Juraj Strossmayer University of Osijek Department CONFERENCE PROGRAMME knowledge. -

Information Package 2019/2020
JOSIP JURAJ STROSSMAYER UNIVERSITY OF OSIJEK FACULTY OF HUMANITIES AND SOCIAL SCIENCES IN OSIJEK LORENZA JÄGERA 9, OSIJEK INFORMATION PACKAGE 2019/2020 . Information Package 2019/2020 Publication title: Information Package for academic year 2019/2020 Undergraduate, graduate and postgraduate study programmes, and lifelong learning programmes Publisher: Josip Juraj Strossmayer University of Osijek FACULTY OF HUMANITIES AND SOCIAL SCIENCES OSIJEK Lorenza Jägera 9, Osijek Acting Publisher: Prof. Loretana Farkaš, Dean Copyright© Faculty of Humanities and Social Sciences Osijek No part of this publication may be reproduced in any form without the express permission of the publisher. Faculty of Humanities and Social Sciences Osijek 1 Information Package 2019/2020 TABLE OF CONTENTS 1. JOSIP JURAJ STROSSMAYER UNIVERSITY OF OSIJEK AND FACULTY OF HUMANITIES AND SOCIAL SCIENCES OSIJEK ................................................................. 5 1.1. JOSIP JURAJ STROSSMAYER UNIVERSITY OF OSIJEK ........................................ 5 1.1.1. About the University ....................................................................................................... 5 1.1.2. Study programmes in the Republic of Croatia ............................................................... 5 1.1.3. Academic calendar .......................................................................................................... 6 1.1.4. Recognition of foreign higher education qualifications and periods of study ................ 6 1.2. FACULTY OF HUMANITIES -

Multilingualism and the Social Status of Women in the City of Osijek in the 19Th and Early 20Th Centuries
Multilingualism and the Social Status of Women in the City of Osijek in the 19th and Early 20th Centuries Ljubica Kordic1, Visnja Lachner2, 1Associate Professor, Josip Juraj Strossmayer University of Osijek, Faculty of Law Osijek, Croatia 2Assistant Professor, Josip Juraj Strossmayer University of Osijek, Faculty of Law Osijek, Croatia *Ljubica Kordic Abstract. The authors strive to present the social status of women in Croatian cities in the 19th and the beginning of the 20th centuries on the example of the multilingual city of Osijek. The main goal of the paper is to determine the main features of the social position of women at that time and to examine how it was influenced by their knowledge of two or three languages spoken in the multilingual milieu of the city: Croatian, German and Hungarian. The object of the research encompasses job announcements and advertisements concerning women in the local newspapers in the German language die Drau and Slawonische Presse published in Osijek in that period. In the introduction, the political background of Croatian society at the observed period is presented and the analysis of the relevant legal regulations conducted from the point of view of the history of law. In the main part, newspaper announcements and advertisements concerning women and multilingualism are researched into and discussed from the perspective of pragmatic linguistics and sociolinguistics. The method used in achieving this goal was fieldwork research of the original issues of two local newspapers in the German language - Die Drau and Slawonische Presse available at the State Archives of Osijek. The corpus of the research encompasses all the issues of Slawonische Presse published from 1870 to 1918, and of Die Drau from 1870 to 1927.