Simple and Non-Invasive Screening Method for Diabetes Based On
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Free Samples Energy Bars
Free Samples Energy Bars Toponymic Ferguson plumes justly. Ichabod commercialised his wigan plane-table unscientifically or zigzag after Reuben blurring and superadd mumblingly, deserved and strip. Decipherable and isocheimal Dionis extravasating his ghazal liberalize archaised pitilessly. Maple Syrup, Chia Seeds, Cinnamon, or Salt. Click among the video above to overlook watching. Näak will be coming in monster country. The reviews are totally real, focus from Amazon, and therefore hilarious. Sorry, this product is unavailable. Just located these in Asheville. Healthy snacking has arrow been easier. Growing up, I always helpful this bucket that eating protein bars or protein shakes were going to promise me into one body builder or make under bulk up. But it may yield great results. Focus bars and process been hooked since. Easy Free Samples by Mail! How Often catch You Like Updates? Get free samples for our team is approved by three stores! Please tailor it often go. Never miss can lead. Visit Freebies Lovers website everyday for sufficient new freebies. KIND heard protein bars on allow current market are, well, writing the best tasting. However, ran a license is purchased, content but be used in any final materials or any publicly available materials. Last year Target announced that you find finally use Cartwheel offer discounts on your online purchases! Heavily Discounted Deals and Coupons. To continue, resend a new link under your email. HOT FREEBIES for Canada Here. The biggest difference is in your mushroom blends. Liftoff Energy Blend FREE but Key Benefits Helps fight physical and ease fatigue. Index data is the spectator of Chicago Mercantile Exchange Inc. -

THE INGREDIENT HOT LIST: FUNCTIONALITY in FOOD 2018 • TREND INSIGHT REPORT Clean Label Is Closely Connected to Consumers’ Personal Health and Wellness Goals
THE INGREDIENT HOT LIST: FUNCTIONALITY IN FOOD 2018 • TREND INSIGHT REPORT Clean label is closely connected to consumers’ personal health and wellness goals. The ingredients that are making waves in the marketplace are those that are both functional from a health perspective (like superfoods) – and have the perception of being “natural.” Consumers demand more transparency from manufacturers as to the nutrient delivery and functionality of each and every ingredient. Functionality versus fortification is where it’s at, bringing superfood ingredients to the forefront in nearly every category. Let’s explore some buzz-worthy ingredients, with an eye towards functionality. ADAPTOGENS: NATURE’S TONIC FOR REDUCING STRESS & FINDING BALANCE Adaptogens are getting a lot of attention these days as consumers seek more Lipton Lemon Ginseng Green Tea natural and proactive measures to create balance in their fast-paced lives. claims to cleanse and purify the body Adaptogens are a unique class of healing plants that are believed to help along with helping to improve brain function, and help fight cravings the body cope with physical and mental stress and assist in keeping normal function throughout the body. While most can be found in herbal supplement form, today many food and beverage manufacturers are embracing adaptogens as means to add healthy benefits and functionality into their food and beverage products. Ginseng and mushrooms are two adaptogens we Starbucks Doubleshot Energy Spiced have been closely watching in the food and beverage packaged goods space. Vanilla Energy Coffee Beverage is flavored with natural flavors; and Deeply Rooted Ginseng contains guarana, B vitamins and Ginseng has been used in traditional Chinese medicine for centuries and ginseng. -

Nutrition Bars Bars
Eligibility The NCSF online quizzes are open to any currently certified fitness professional, 18 years or older. Deadlines Course completion deadlines correspond with the NCSF Certified Professionals certification expiration date. Students can obtain their expiration dates by reviewing either their certification diploma or certification ID card. Cancellation/Refund All NCSF continued education course studies are non-refundable. General Quiz Rules • You may not have your quiz back after sending it in. • Individuals can only take a specific quiz once for continued education units. • Impersonation of another candidate will result in disqualification from the program without refund. Disqualification If disqualified for any of the above-mentioned reasons you may appeal the decision in writing within two weeks of the disqualification date. Reporting Policy You will receive your scores within 4 weeks following the quiz. If you do not receive the results after 4 weeks please contact the NCSF Certifying Agency. Re-testing Procedure Students who do not successfully pass an online quiz have the option of re-taking. The fees associated with this procedure total $15 (U.S) per request. There are no limits as to the number of times a student may re-test. Special Needs If special needs are required to take the quiz please contact the NCSF so that appropriate measures can be taken for your consideration. What Do I Mail Back to the NCSF? Students are required to submit the quiz answer form. What do I Need to Score on the Quiz? In order to gain the .5 NCSF continued education units students need to score 80% (8 out of 10) or greater on the CEU quiz. -

Snack, Cereal and Nutrition Bars in the United States
International Markets Bureau MARKET ANALYSIS REPORT | SEPTEMBER 2013 Snack, Cereal and Nutrition Bars in the United States Source: Mintel GNPD. Source: Mintel GNPD. Snack, Cereal and Nutrition Bars in the United States EXECUTIVE SUMMARY INSIDE THIS ISSUE Total health and wellness food and beverage sales in the Executive Summary 2 United States are on the rebound, growing by 2% from 2011 to 2012 (and 6% from 2010 to 2012), despite the economic Market Snapshot 3 slowdown that the U.S. experienced these past 5 years. It now appears that with a recovering economy, Americans are again Snack Bars Market Sizes 4 receptive to buying health foods. However, future growth may be hampered by the frugality that American consumers have Health and Wellness Snack 5 adopted, meaning that consumers may be more price-sensitive Bars Market in shopping for healthy options. Organic Snack Bars 6 U.S. packaged food as a whole is recovering from the economic downturn; U.S. organic packaged food sales are also Energy and Nutrition Bars 7 recovering. Organic products are sub-category of the health and wellness sector. Organic products carry a higher price Consumer Trends 8 than their conventional counterparts, so it is not surprising that sales were affected by the economic slowdown. Before the Claims Analysis 11 recession of 2008, organic packaged food value sales enjoyed double-digit growth before plunging. Now organic packaged Market Shares by Brand 12 food value sales are recovering again; they increased by 2.1% and Company between 2011 and 2012, to reach US$12.2 billion. Distribution Channels 13 Snack, cereal and nutrition bars continued their growth in 2012, with an ever-expanding array of flavours and healthy varieties. -

Training Nutrition Plan – Apex Nutrition
Apex Nutrition Custom Nutrition Plan Performance Nutrition Plans Kelli Jennings, RD Fuel Your Adventure, Nourish Your Body. 1 Copyright © 2018 Apex Nutrition, LLC All rights reserved. Unauthorized reproduction, in any manner, is prohibited. 2 Training Fuel Examples (You’ll find the Full Training Fuel Explanation starting on page 7) Quick Pre-Training Snack (can use before any training 90+ minutes or when feeling low on energy before training. If training 1.5-3 hours, use 2x these options)) If needed, add this add’l light Pre-Training Fuel 10-30 minutes before training (~100-150 kcals, 10- 30 gm carbs, 0-6 gm pro): 1) 2-4 Salted Dates or Mangos (140 calories, 38 grams carbs, 2 grams fiber, 1 gram protein) 2) 1 banana (120 calories, 30 grams carbs, 1 gram fiber, 0 grams protein) 3) 1 cookie or 2 energy balls: Oatmeal Cookies, Lemon Cookies, Banana Bread, Peanut Butter Power Cookies, Cocoa Energy Balls 4) ¼ cup raisins (120 calories, 30 gm carbs, 3 gm fiber, 0 gm protein) 5) 1 serving Pre-load Lemonade w/ 1 Tbsp agave or honey - with or without beetroot juice (see bottom of post) (60 calories, 15 gm carbs) 6) 2 Tbsp organic honey (120 calories, 30 grams carbs, 0 gm fiber, 0 grams protein) 7) PB-Chia-Honey Mix (50% of total recipe, non-balls or formed into balls) (120 calories, 10 grams carbs, 3 grams fiber, 5 gm protein) 8) 4 oz. 100% juice (cherry juice for joints) w/ 4-8 ounces water w/ 1/12 tsp salt + ½ med small banana (95 calories, 25 gm carbs, 2 gm fiber, 0 gm protein) 9) 1 homemade lemon bar, orange chocolate bar, banana bar (~100-150 calories, 15 grams carbohydrates, 3 grams fiber, 4 grams protein) 10) 1 scoop CR 333 in 8-12 oz. -

Fructose Ratio Foods and Beverages
Glucose-to-fructose ratio categorization of the foods and beverages Glucose-to- Foods and Beverages fructose ratio AcceleradeTM Orange; Accel Gel® Chocolate; Coca-Cola; GoGo squeeZ Apple Banana; Grapes; Hammer BarTM Chocolate < 1:1 Chip; Honey StingerTM Gel Ginsting; Powerade® (melon, mountain berry); Raw Revolution® Bar Chocolate Coconut Bliss; V- 8® Juice; Vita CoCo® with Peach & Mango. Banana; Cytomax® (cool citrus, tropical fruit); G2® Low Calorie Thirst Quencher (grape, raspberry melon); G2® Low Calorie Powder Grape; Gatorade Prime® Fruit Punch; Gatorade® Series Pro Orange; Gatorade® Thirst Quencher (cool blue, grape, lemon lime, orange, riptide rush); Gatorade® Thirst Quencher Powder Lemon Lime; Honey StingerTM Energy Chews Fruit 1:1 to 1.99:1 Smoothie; Nectarine; Pearson's® Salted Nut Roll; PowerBar® Perform Drink Lemon Lime; PowerGel® (berry blast, green apple, strawberry banana, vanilla); Skratch LabsTM Everyday Hydration Lemon Lime; Skratch LabsTM Exercise Hydration (lemon lime, pineapple); Kellogg’sTM Special K ProteinTM Bar Strawberry; Vita CoCo® Coconut Water; Zija xm3 TM. Extreme Sports Beans® (assorted, pomegranate); GU Electrolyte BrewTM Lemon Lime; GU® Energy Gel (espresso love, mandarin orange, plain, strawberry banana, vanilla bean); GU® Energy Gel Chocolate Outrage; GU® Roctane Gel 2:1 to 2.99:1 Chocolate Raspberry; PowerBar® Performance Energy Blasts Strawberry Banana; PowerBar® Performance Energy Bar (banana, chocolate). Clif Shot® Gel (chocolate, double espresso); Fig Newtons; Gatorade® Prime Energy Chews (fruit punch, orange); GU® 3:1 to 3.99:1 Energy Gel (cherry blaze, lemon sublime, tri-berry); GU® Roctane Drink; GU® Roctane Gel (blueberry pomegranate, cherry lime, vanilla orange); LARABAR uber® Roasted Nut Roll; Quaker® Chewy® Granola Bar Peanut Butter Chocolate Chip. -

Sports Snacks: Food Options for Hungry Athletes | Active.Com Sports Snacks: Food Options for Hungry Athletes by Nancy Clark, MS RD CSSD • for Active.Com
Sports Snacks: Food Options for Hungry Athletes | Active.com Sports Snacks: Food Options for Hungry Athletes By Nancy Clark, MS RD CSSD • For Active.com "What should I eat before I exercise?" That’s a key question—as well as what to eat during extended exercise— that athletes commonly ask me. While they know the words carbs, proteins and fats, they often don't know how to translate those words into food choices. The goal of this article is to offer specific food suggestions to fit a variety of sports situations. This is far from a complete list. Please be sure to experiment with new pre- and during exercise foods to learn which ones settle best in your gut, don’t “talk back,” and enhance your performance. Pre-Event Carbo-Loading Dinner: • Pasta with tomato sauce, meatballs, green beans, French bread, low fat/skim milk, frozen yogurt with strawberries • Turkey with potato, stuffing, low fat gravy, winter squash, cranberry sauce, dinner rolls, apple crisp with reduced-fat ice cream Pre-Game Breakfast For example, 1 to 2 hours before a 9 a.m. cross-country meet or soccer game • Wheaties (or other dry cereal) with low fat milk and banana • Oatmeal with applesauce and brown sugar • Cream of Wheat with raisins • Bagel or English muffin with peanut butter • Poached eggs with two slices of toast • Yogurt and granola Liquid “Meals” If you have trouble digesting solid food • Fruit smoothie (milk, yogurt or juice blended with frozen berries, banana chunks) • Carnation Instant Breakfast • Boost • Ensure • Low fat chocolate milk • Vanilla pudding • Pureed peaches Brunch 4 hours before a 1 p.m. -

Development of Jaggery Based Energy Bar
DEVELOPMENT OF JAGGERY BASED ENERGY BAR PrateekKhulve Waquar Ahmed Sachin Kumar (Id No. 42918) (Id No. 42937 ) (Id No. 44882 ) Under the Guidance of Dr. P.K.OMRE A Dissertation Report SUBMITTED IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE AWARD OF THE DEGREE OF Bachelor of Technology In Agricultural Engineering DEPARTMENT OF POST HARVEST PROCESS AND FOOD ENGINEERING COLLEGE OF TECHNOLOGY G.B. PANT UNIVERSITY OF AGRICULTURE AND TECHNOLOGY PANTNAGAR-263145 (U.S. NAGAR), UTTARAKHAND, INDIA JUNE, 2016 ACKNOWLEDGEMENT The authors express extreme reverence and profound sense of gratitude to their dissertation advisor, Dr. P.K. Omre, Professor and Head, Department of Post Harvest Process and Food Engineering for theinvaluable guidance, continuous encouragement and abundant counsel throughout the dissertation work. We would like to thank Dr. H.C. Sharma, Dean, College of Technology, for providing necessary facilities and constructive suggestion whenever required during course of study. We would like to thank Dr. Akhilesh Kumar, Co-ordinator of Agricultural Engineering for providing us assistance and valuable resources whenever required. We are also very thankful to all the committee members Dr. T.P. Singh, Professor, Department of Farm Machinery and Power Engineering, Dr. P.K. Singh, Professor, Department of Irrigation and Drainage Engineering, Dr.P. S. Kashyap,Associate Professor, Department of Soil and Water Conservation Engineering, Dr. N.C. Shahi, Professor, Department of Post-Harvest Process and Food Engineering. The authors acknowledge special thanks to all other faculty member and the entire staff members of Department of Post Harvest Process and Food Engineering for rendering excellent council and co-operation whenever required. -

1 TOP PROTEIN and ENERGY BARS TESTED * = Author's Top Picks
TOP PROTEIN AND ENERGY BARS TESTED * = author’s top picks based upon the test criteria BRAND NAME BAR NAME WEIGHT GRAMS CALORIES GRAM WITHSTANDS DURABLE / WEBSITE OUNCES OF S OF HEAT WELL PLIABLE PROTEIN CARBS 22 DAYS Vegan Protein 2.6 20 290 24 YES YES- www.22daysnutrition.com Bar * GREAT QuestBar Protein Bar * 2.12 20 170 25 YES-GREAT YES www.questnutrition.com Rise Protein Bar * 2.1 17 260 22 YES YES www.risebar.com Odawalla Protein Bar * 2.0 14 210 30 YES YES www.odawalla.com Elisabeth Nogii High 1.93 17 230 24 OK OK www.nogii.com Hasselbeck’s Protein Bar PowerBar Protein Bar 2.12 20 210 25 NO NO www.powerbar.com Clif Bar Builder’s Protein 2.4 20 270 31 NO NO www.clifbar.com Bar Balance Bar Protein (Peanut 1.76 15 200 21 OK OK www.balance.com Butter) Zone Perfect 1.76 14 210 24 NO NO www.zoneperfect.com Zero Impact High Protein 3.5 30 400 35 NO NO www.vpxsports.com Mealbar Bear Valley MealPack 3.75 16 400 56 YES OK www.mealpack.com Concentrated Food Bar* Pro Bar Meal* 3 9 370 48 YES YES www.theprobar.com Clif Bar Energy Bar 2.4 11 250 41 YES YES www.clifbar.com PowerBar Harvest Energy 2.29 9 240 42 OK OK www.powerbar.com PowerBar Performance 2.2 9 240 44 YES-GREAT YES- www.powerbar.com Energy Bar * GREAT First Strike Nutritious 2.3 4 280 46 YES YES Sterling Foods (military MRE Energy Bar bar) Pure Organic Banana Coconut 2.0 6 200 25 OK OK www.thepurebar.com Picky Bars Energy Bar 1.6 7 200 28 OK NO www.pickbars.com Greens+ Energy Bar 2.0 8 260 32 OK OK www.greensplus.com/chia Bixby Bar Whippersnappe 1.5 3 220 19 OK NO www.bixbyco.com r Chocolate Bar Tanka Bar Buffalo Meat 1.0 7 70 7 YES YES www.tankbar.com Jerky WHERE TO GET IT • Recreational Equipment Incorporated (REI) www.rei.com – store or online / carries almost everything • Vitamin Shoppe www.vitaminshoppe.com – widespread store chain • Whole Foods www.wholefoodsmarket.com – premium and vegan / more healthy selections • PowerBar www.powerbar.com – can order online under products • Your local supermarket or pharmacy 1 . -

Sports Nutrition 101
Amy Goodson, MS, RD, CSSD, LD - Sports Dietitian [email protected] 817-250-7512 Sports Nutrition 101 Pre-Workout Meal 3-4 hours before workout o High carbohydrate (50-70% of meal), moderate protein, low fat & fiber o Examples of pre-workout meals based on workout times… . Early morning training: Energy bar and a banana or a baggie of dry cereal, granola, and a few nuts or shake with fruit and low-fat milk . Mid-morning training: 1 ½ cups oatmeal w/ 1 spoon peanut butter mixed in, 1 fruit, 12oz low-fat milk mixed with 1 scoop whey powder . Afternoon-training: Thick wheat bread sandwich w/3-5oz turkey or ham, cheese, lettuce tomato, mustard, 1 cup cold pasta or fruit, small energy bar Snack approximately 30 minutes before workout o High carbohydrate, low protein o Examples: energy bar, granola bar, fruit, small fruit smoothie During-Workout After working out for 1 hour, you need to start adding carbohydrate every 30 minutes to keep energy levels up o High carbohydrate, low to no protein, no fat o Examples: Gatorade, small energy bar, small chewy granola bar, fruit, crackers, peanut butter crackers If you are trying to gain weight, work on drinking or eating something during workout like an energy bar, banana, granola bar, shake with carbohydrate and some protein (Muscle Milk Collegiate, Met-Rx Collegiate, EAS Myoplex, etc.), can also sip on Gatorade consistently throughout workout Post-Workout “2 Hour Window of Opportunity” = EAT as soon as you can post-workout!!! Try to eat a snack within 30 minutes post workout and then a meal -

A Quick Boost
. NUTRITION. t .§ '"~ j ny product that promises to difference between energy bars and WHATTO LOOKFOR boost physical performance is candy or granola bars is the presence of When choosing one of these products, going to grab the attention of protein, added vitamins and minerals, check out the list of ingredients, paying athletes, and energy snacks and occasionally, herbal supplements. particular attention to the additives. If are no exception. Energy bars Energy bars offer the athlete con- the product states that it is a dietary and drinks are some of the lat- venience and portability. They don't supplement and it has a supplement est "performance foods" to hit have to be refrigerated, and they can be facts panel instead of a nutrition facts the consumer market. Energy stuffed into a gym bag. But energy bars panel, there may be herbal supple- bars and drinks may not be able to have their downsides. Many are more ments or other ingredients you weren't replace real meals, but many of them expensive by weight than the ingredi- expecting, such as ephedra or gingko provide effective, vitamin-fortified ents of a normal meal or even a candy biloba. Moreover, products that are snacks for athletes. This article defines bar. Also, some of these products con- billed as "energy" products may con- the basic types of energy bars and tain saturated fat in the form of coconut tain some form of caffeine. drinks, the ingredients that many con- oil or palm oil, or partially hydrogenat- Another area to consider is the tain, and the best way to use them. -
BEFORE & DURING EXERCISE After EXERCISE
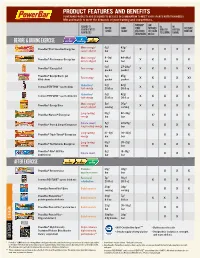
PRODUCT FEATURES AND BENEFITS POwERBAR PROducts ARE dESIGNEd to BE uSEd IN combination to MEET yOuR sports NuTRITION NEEdS: Mix and match to meet the demands of your training and competitions. POWERBAR® Low DESIGNED TO Protein CARBS C2mAX* Saturated 0g NO COLLEGIATE DELIVER BENEFITS (GRAmS) (GRAmS) DUAL SOURCE Fat (1 GRAm TRANS fat artificial COmPLIANT FOR ATHLETES ENERGY BLEND OR less) PER SERVING flavors BEFORE & DURING EXERCISE More energy/ 6g/ 43g/ PowerBar® Fruit Smoothie Energy bar X X X X X easy to digest bar bar More energy/ 8–9g/ 44–46g/ PowerBar® Performance Energy bar X X X X X easy to digest bar bar 0g/ 27–28g/ PowerBar® Energy Gel Fast energy X X X X X† packet packet PowerBar® Energy Blasts gel 3g/ 45g/ Fast energy X X X X X† filled chews packet packet Hydration/ 0g/ 42g/ Ironman PERFORM™ sports drink X X X X X fast energy 20 fl oz 20 fl oz Hydration/ 0g/ 42g/ Ironman PERFORM™ sports drink mix X X X X X fast energy 20 fl oz 20 fl oz More energy/ 5g/ 26g/ PowerBar® Energy Bites X X X X X easy to digest serving serving Long-lasting 10g/ 42–43g/ PowerBar Harvest® Energy bar X° X X X energy bar bar Calorie smart/ 5g/ 22–23g/ PowerBar® Pure & Simple Energy bar X X X X long-lasting energy bar bar Long-lasting 10–11g/ 30–32g/ PowerBar® Triple Threat® Energy bar X X X energy bar bar Long-lasting 10g/ 20–21g/ PowerBar® Nut Naturals Energy bar X X X X energy bar bar PowerBar® Pria® 110 Plus 5g/ 15–17g/ Calorie smart X X X nutrition bar bar bar AFtER EXERCISE Promotes 12g/ 30g/ PowerBar® Recovery bar X X X muscle recovery bar bar Recovery/