A Short Term Study of Dissolved Oxygen Behavior in Adyar River, Chennai
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Altis Sri Lakshmi Vilas - Kotturpuram, Chennai Ultimate Abode for You and Your Loving Family
https://www.propertywala.com/altis-sri-lakshmi-vilas-chennai Altis Sri Lakshmi Vilas - Kotturpuram, Chennai Ultimate abode for you and your loving family. Altis Sri Lakshmi Vilas by Altos Properties at Kotturpuram in Chennai offers residential project that host 3 bhk and 4 bhk apartment in various sizes. Project ID: J396214118 Builder: Altis Properties Location: Altis Sri Lakshmi Vilas,Chitra Nagar, Kotturpuram, Chennai (Tamil Nadu) Completion Date: Jul, 2020 Status: Started Description Altis Sri Lakshmi Vilas built in a contemporary style and comprising 11 Luxury 3 bhk and 4 bhk apartments in the size ranges in between 2300 to 4281 sqft. it is located in the most sought after residential neighborhood - Kotturpuram. The compact apartment complex comes with its own exclusive gym, lounge and rooftop barbecue area. There are number of benefits of living in Apartments with a good locality. The location of the project makes sure that the home-seekers are choosing the right Apartments for themselves. Amenities: Landscaped Garden Gymnasium Play Area Intercom Gated community Security 24Hr Backup Electricity Altis Properties is an eminent name in the real estate market of Chennai. It strives to revolutionize the way homes, offices, and social spaces are built. The company is known for building a successful range of premium and luxury homes with a commitment to on-time delivery and solid build quality. It works hard to maximize customer delight by exceeding their expectations at every level, while also striving to deliver excellent value to its stakeholders. Ecstasea at Muttukadu, Horizon at Saligramam, and Urbanville at Velachery are some of the company’s prestigious projects. -

Zooplankton Diversity of Freshwater Lakes of Chennai, Tamil Nadu with Reference to Ecosystem Attributes
International Journal of Int. J. of Life Science, 2019; 7 (2):236-248 Life Science ISSN:2320-7817(p) | 2320-964X(o) International Peer Reviewed Open Access Refereed Journal Original Article Open Access Zooplankton diversity of freshwater lakes of Chennai, Tamil Nadu with reference to ecosystem attributes K. Altaff* Department of Marine Biotechnology, AMET University, Chennai, India *Corresponding Author: [email protected] Manuscript details: ABSTRACT Received: 18.04.2019 Zooplankton diversity of twelve water bodies of Chennai with reference to Accepted: 05.05.2019 variation during pre-monsoon, monsoon, post-monsoon and summer Published: 20.06.2019 seasons is investigated and reported. Out of 49 zooplankton species recorded, 27 species belonged to Rotifera, 10 species to Cladocera, 9 Editor: Dr. Arvind Chavhan species to Copepoda and 3 species to Ostracoda. The Rotifers dominated compared to all other zooplankton groups in all the seasons. However, the Cite this article as: diversity of zooplankton varied from season to season and the maximum Altaff K (2019) Zooplankton diversity was recorded in pre- monsoon season while minimum was diversity of freshwater lakes of observed in monsoon season. The common and abundant zooplankton in Chennai, Tamil Nadu with reference these water bodies were Brachionus calyciflorus, Brchionus falcatus, to ecosystem attributes, Int. J. of. Life Brachionus rubens, Asplancna brightwelli and Lecane papuana (Rotifers), Science, Volume 7(2): 236-248. Macrothrix spinosa, Ceriodaphnia cornuta, Diaphnosoma sarsi and Moina micrura (Cladocerans), Mesocyclops aspericornis Thermocyclops decipiens Copyright: © Author, This is an and Sinodiaptomus (Rhinediaptomus) indicus (Copepods) and Stenocypris open access article under the terms major (Ostracod). The density of the zooplankton was high during pre- of the Creative Commons Attribution-Non-Commercial - No monsoon and post-monsoon period than monsoon and summer seasons. -

Thiruvallur District
DISTRICT DISASTER MANAGEMENT PLAN FOR 2017 TIRUVALLUR DISTRICT tmt.E.sundaravalli, I.A.S., DISTRICT COLLECTOR TIRUVALLUR DISTRICT TAMIL NADU 2 COLLECTORATE, TIRUVALLUR 3 tiruvallur district 4 DISTRICT DISASTER MANAGEMENT PLAN TIRUVALLUR DISTRICT - 2017 INDEX Sl. DETAILS No PAGE NO. 1 List of abbreviations present in the plan 5-6 2 Introduction 7-13 3 District Profile 14-21 4 Disaster Management Goals (2017-2030) 22-28 Hazard, Risk and Vulnerability analysis with sample maps & link to 5 29-68 all vulnerable maps 6 Institutional Machanism 69-74 7 Preparedness 75-78 Prevention & Mitigation Plan (2015-2030) 8 (What Major & Minor Disaster will be addressed through mitigation 79-108 measures) Response Plan - Including Incident Response System (Covering 9 109-112 Rescue, Evacuation and Relief) 10 Recovery and Reconstruction Plan 113-124 11 Mainstreaming of Disaster Management in Developmental Plans 125-147 12 Community & other Stakeholder participation 148-156 Linkages / Co-oridnation with other agencies for Disaster 13 157-165 Management 14 Budget and Other Financial allocation - Outlays of major schemes 166-169 15 Monitoring and Evaluation 170-198 Risk Communications Strategies (Telecommunication /VHF/ Media 16 199 / CDRRP etc.,) Important contact Numbers and provision for link to detailed 17 200-267 information 18 Dos and Don’ts during all possible Hazards including Heat Wave 268-278 19 Important G.Os 279-320 20 Linkages with IDRN 321 21 Specific issues on various Vulnerable Groups have been addressed 322-324 22 Mock Drill Schedules 325-336 -
S.No. Shop Address 1 Anna Nagar Shanthi Colony
S.No. Shop Address Anna Nagar Shanthi Colony Aa-144, 2nd Floor, 3rd Avenue, (Next To Waves) Anna Nagar, Ch-600040. 1 Anna Nagar West No 670,Sarovar Building, School Road, Anna Nagar West, Chennai - 600101. 2 Mogappair East 4/491, Pari Salai, Mogappair East, (Near Tnsc Bank) Ch-600037 3 Mogappair West 1 Plot No.4, 1st Floor, Phase I, Nolambur,(Near Reliance Fresh) Mogappair 4 West, Ch-600037. Annanagar West Extn Plot No: R48, Door No - 157, Tvs Avenue Main Road,Anna Nagar West 5 Extension,Chennai - 600 101. Opp To Indian Overseas Bank. Red Hills 1/172a, Gnt Road, 2nd Floor, Redhills-Chennai:52. Above Lic, Next To Iyappan 6 Temple K.K.Nagar 2 No.455, R.K.Shanuganathan Road, K K Nagar, Land Mark:Near By K M 7 Hospital, Chennai - 600 078 Tiruthani No. 9, Chittoor Road, Thirutani - 631 209 8 Anna Nagar (Lounge) C Block, No. 70, Tvk Colony, Annanagar East, Chennai - 102. 9 K.K.Nagar 1 Plot No 1068, 1st Floor, Munuswami Salai, (Opp To Nilgiri Super Market) 10 K.K.Nagar West, Ch-600078. Alapakkam No. 21, 1st Floor, Srinivasa Nagar,Alapakkam Main 11 Road,Maduravoyal,Chennai 600095 Mogappair West 2 No-113, Vellalar Street, Mogappair West, Chennai -600 037. 12 Poonamalle # 35, Trunk Road, Opp To Grt Poonamalle Chennai-600056. 13 Karayanchavadi N0. 70, Trunk Road, Karayanchavadi, Poonamallee, Chennai - 56 14 Annanagar 6th Avenue 6th Avenue,Anna Nagar,Chennai 15 Chetpet Opp To Palimarhotel,73,Casamajorroad,Egmore,Ch-600008 16 Egmore Lounge 74/26,Fagunmansion,Groundfloor,Nearethirajcollege,Egmore,Chennai-600008 17 Nungambakkam W A-6, Gems Court, New.25 (Old No14), Khader Nawaz Khan Road, (Opp Wills 18 Life Style) Nungambakkam, Ch-600034. -

29 Dec 2016 150913530ZNT6I
1. Performance monitoring of all STPs in Chennai, especially those commissioned under NRCP reflecting the impact of CCRCP. During 2000-01, the Government of Tamil Nadu, along with the Government of India formulated the Chennai City River Conservation Project (CCRCP) with an objective to: i. Prevent sewage entering into the waterways. ii. Augment the treatment capacity of the Sewage Treatment Plants at Nesapakkam, Koyambedu, Kodungaiyur and Perungudi. iii. Keep the city waterways clean on a sustainable basis. The Ministry of Environment & Forests, Government of India during the year 2000-01 had sanctioned a sum of Rs.491.52 crore for the component “Prevention of untreated sewage flow reaching waterways in Chennai City”, which was carried out through Chennai Metropolitan Water Supply and Sewerage Board (CMWSSB) under NRCP. The following are the major components: S.No. Work Components Amount (Crore) 1 Strengthening & Capacity expansion of sewer system 382.00 2 Construction of STPs 109.52 Total 491.52 CCRCP was commenced and implemented by CMWSSB from the year 2001 in 16 packages. These works were implemented not just in the Cooum River Basin, but also in Adyar River Basin, Captain Cotton Canal Basin, Otteri Nallah Basin, Buckingham Canal Basin and in Mambalam Drain Basin. The project consisted of laying of interceptors for a length of 59.2 km, improvements to 28 pumping stations, construction of 3 new pumping stations, laying of sewage pumping mains for a length of 28.85 km and gravity conveying mains for a length of 17.3 km through 12 packages. Construction of 4 sewage treatment plants to enhance the sewage treatment capacity by 264 MLD was carried out in Packages 13 to 16. -

Tamil Nadu Government Gazette
© GOVERNMENT OF TAMIL NADU [Regd. No. TN/CCN/467/2009-11. 2009 [Price: Rs. 16.80 Paise. TAMIL NADU GOVERNMENT GAZETTE PUBLISHED BY AUTHORITY No. 21] CHENNAI, WEDNESDAY, JUNE 3, 2009 Vaikasi 20, Thiruvalluvar Aandu–2040 Part VI—Section 4 Advertisements by private individuals and private institutions. CONTENTS PRIVATE ADVERTISEMENTS Pages Change of Names .. .. 767-808 Notices .. .. NOTICE NO LEGAL RESPONSIBILITY IS ACCEPTED FOR THE PUBLICATION OF ADVERTISEMENTS REGARDING CHANGE OF NAME IN THE TAMIL NADU GOVERNMENT GAZETTE. PERSONS NOTIFYING THE CHANGES WILL REMAIN SOLELY RESPONSIBLE FOR THE LEGAL CONSEQUENCES AND ALSO FOR ANY OTHER MISREPRESENTATION, ETC. (By Order) Director of Stationery and Printing. CHANGE OF NAMES I, K. Kamaladevi, daughter of Thiru C. Karthikeyan, born I, P. Rajeswari, wife of Thiru P. Ravi, born on 28th July on 24th May 1982 (native district: Madurai), residing at 1968 (native district: Karur), residing at No. F9, TNPL Colony, No. 633, Kurinji Street, Gomathipuram, Malamadai Kagithapuram Post, Karur-639 136, shall henceforth be known Panchayat, Madurai-625 020, shall henceforth be known as as P SOWJANYA. C.K. KAMALADEVI. P. RAJESWARI. K. KAMALADEVI. Kagithapuram, 25th May 2009. Madurai, 25th May 2009. My son, S. Sanjaikumar, born on 20th June 2006 (native My son, V. Ragunath, born on 15th May 2000 (native district: Karur), residing at No. 15/7A/7, Thotta Street, district: Salem), residing at No. 1-1/257, Kurinchi Nagar, Vadivelampalayam, Nanaparappu, Pugalur SF Post, Mohan Nagar Post, Salem-636 030, shall henceforth be Karur-639 113, shall henceforth be known as S SUJEET. known as V. SHREENATH. P. SADHASIVAM. KN. -
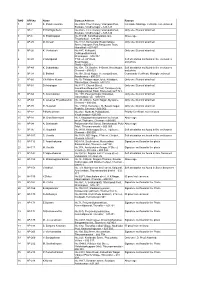
SNO APP.No Name Contact Address Reason 1 AP-1 K
SNO APP.No Name Contact Address Reason 1 AP-1 K. Pandeeswaran No.2/545, Then Colony, Vilampatti Post, Intercaste Marriage certificate not enclosed Sivakasi, Virudhunagar – 626 124 2 AP-2 P. Karthigai Selvi No.2/545, Then Colony, Vilampatti Post, Only one ID proof attached. Sivakasi, Virudhunagar – 626 124 3 AP-8 N. Esakkiappan No.37/45E, Nandhagopalapuram, Above age Thoothukudi – 628 002. 4 AP-25 M. Dinesh No.4/133, Kothamalai Road,Vadaku Only one ID proof attached. Street,Vadugam Post,Rasipuram Taluk, Namakkal – 637 407. 5 AP-26 K. Venkatesh No.4/47, Kettupatti, Only one ID proof attached. Dokkupodhanahalli, Dharmapuri – 636 807. 6 AP-28 P. Manipandi 1stStreet, 24thWard, Self attestation not found in the enclosures Sivaji Nagar, and photo Theni – 625 531. 7 AP-49 K. Sobanbabu No.10/4, T.K.Garden, 3rdStreet, Korukkupet, Self attestation not found in the enclosures Chennai – 600 021. and photo 8 AP-58 S. Barkavi No.168, Sivaji Nagar, Veerampattinam, Community Certificate Wrongly enclosed Pondicherry – 605 007. 9 AP-60 V.A.Kishor Kumar No.19, Thilagar nagar, Ist st, Kaladipet, Only one ID proof attached. Thiruvottiyur, Chennai -600 019 10 AP-61 D.Anbalagan No.8/171, Church Street, Only one ID proof attached. Komathimuthupuram Post, Panaiyoor(via) Changarankovil Taluk, Tirunelveli, 627 761. 11 AP-64 S. Arun kannan No. 15D, Poonga Nagar, Kaladipet, Only one ID proof attached. Thiruvottiyur, Ch – 600 019 12 AP-69 K. Lavanya Priyadharshini No, 35, A Block, Nochi Nagar, Mylapore, Only one ID proof attached. Chennai – 600 004 13 AP-70 G. -

Urban and Landscape Design Strategies for Flood Resilience In
QATAR UNIVERSITY COLLEGE OF ENGINEERING URBAN AND LANDSCAPE DESIGN STRATEGIES FOR FLOOD RESILIENCE IN CHENNAI CITY BY ALIFA MUNEERUDEEN A Thesis Submitted to the Faculty of the College of Engineering in Partial Fulfillment of the Requirements for the Degree of Masters of Science in Urban Planning and Design June 2017 © 2017 Alifa Muneerudeen. All Rights Reserved. COMMITTEE PAGE The members of the Committee approve the Thesis of Alifa Muneerudeen defended on 24/05/2017. Dr. Anna Grichting Solder Thesis Supervisor Qatar University Kwi-Gon Kim Examining Committee Member Seoul National University Dr. M. Salim Ferwati Examining Committee Member Qatar University Mohamed Arselene Ayari Examining Committee Member Qatar University Approved: Khalifa Al-Khalifa, Dean, College of Engineering ii ABSTRACT Muneerudeen, Alifa, Masters: June, 2017, Masters of Science in Urban Planning & Design Title: Urban and Landscape Design Strategies for Flood Resilience in Chennai City Supervisor of Thesis: Dr. Anna Grichting Solder. Chennai, the capital city of Tamil Nadu is located in the South East of India and lies at a mere 6.7m above mean sea level. Chennai is in a vulnerable location due to storm surges as well as tropical cyclones that bring about heavy rains and yearly floods. The 2004 Tsunami greatly affected the coast, and rapid urbanization, accompanied by the reduction in the natural drain capacity of the ground caused by encroachments on marshes, wetlands and other ecologically sensitive and permeable areas has contributed to repeat flood events in the city. Channelized rivers and canals contaminated through the presence of informal settlements and garbage has exasperated the situation. Natural and man-made water infrastructures that include, monsoon water harvesting and storage systems such as the Temple tanks and reservoirs have been polluted, and have fallen into disuse. -

Analysis of Sewage Water from Cooum River in Chennai
International Journal of Pure and Applied Mathematics Volume 116 No. 13 2017, 123-129 ISSN: 1311-8080 (printed version); ISSN: 1314-3395 (on-line version) url: http://www.ijpam.eu Special Issue ijpam.eu ANALYSIS OF SEWAGE WATER FROM COOUM RIVER IN CHENNAI 1Aswathy.M, 2Gautam Kumar, 3Dilip Kumar Thakur 1,2,3 Department of Civil Engineering, BIST, Bharath Institute of Higher Education and Research (BIHER), Bharath University, Chennai. [email protected], [email protected], [email protected] Abstract: The present study assesses the sewage water physiological activities and life processes of aquatic of cooum river (Saidapet) Chennai, Tamil Nadu, India. organisms are generally influenced by such polluted five water samples were taken on different timings. waste and hence[1-7], it is essential to study physico- Some sample were collected from logged water and chemical characteristics of water. The following are the some samples were collected from perennial flow of parameter analysed to assess the water quality: water. Water is an indispensable natural resource on Physical parameters: Colour, Temperature, Turbidity earth. Safe drinking water is the primary need of every and Odour. Chemical parameters: pH, Electrical human being. The quality of water is of vital concern Conductivity (E.C), Total Solids (TS), Total Dissolved for mankind since it is linked with human health, Solids (TDS), Total Suspended Solids (TSS), protection of the environment, plant growth and Alkalinity, Total Hardness, Calcium Hardness, sustainable development. River water is used in huge Magnesium Hardness, Nitrates, Phosphates, Sulphates, amount for domestic purpose, industrial purpose, and Chlorides, Residual Chloride, Dissolved Oxygen agriculture purpose and also the water were treated in (D.O), Biological Oxygen Demand (BOD), Chemical water treatment plant and used for drinking and many Oxygen Demand (COD), Fluorides, Free Carbon- other purpose. -
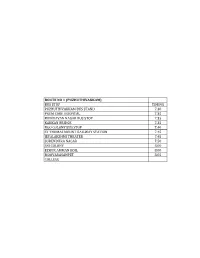
Puzhuthivakkam
ROUTE NO 1 (PUZHUTHIVAKKAM) BUS STOP TIMING PUZHUTHIVAKKAM BUS STAND 7.30 PREM CARE HOSPITAL 7.35 BRINDAVAN NAGAR BUS STOP 7.35 KAKKAN BRIDGE 7.35 NGO COLONY BUS STOP 7.40 ST THOMAS MOUNT RAILWAY STATION 7.45 JEYALAKSHMI THEATER 7.45 SURENDHRA NAGAR 7.50 SBI COLONY 8.00 EZHUR AMMAN KOIL 8.00 MOOVARASANPET 8.05 COLLEGE ROUTE NO 3 (VINAYAGAPURAM - RETTERY) BUS STOP TIMING NADHAMUNI THEATER(VILLIVAKKAM) 6.20 VRJ HOSPITAL 6.20 SENTHIL NAGAR 6.25 VINAYAGAPURAM BUS STOP 6.30 RETTERY SIGNAL 6.35 KOLATHUR MOOGAMBIGAI SHOP 6.35 WELDING SHOP BUS STOP 6.35 DON BOSCO SCHOOL 6.40 PERAVALLUR BUS STOP 6.40 GANAPATHY STORES (PERAVALLUR JUNCTION ) 6.40 AGARAM GANDHI STATUE 6.45 VENUS GANDHI STATUE 6.45 PERAMBUR BRIDGE PETROL BUNK 6.50 OTTERI BRIDGE BUS STOP (ESI CUT) 6.55 AYANAVARAM SIGNAL 7.00 AYANAVARAM ESI HOSPITAL 7.00 PURASAIWAKKAM WATER TANK(ICICI BANK)NEAR 7.05 PACHIYYAPAS COLLEGE 7.10 METHA NAGAR 7.10 CHOLAI MEDU BUS STOP 7.15 LOYOLA COLLEGE 7.15 LIBERTY NEAR STATE BANK 7.25 DURAISAMY SUBWAY JUNCTION 7.30 AYODHYAMANDAPAM BUS STOP 7.30 POSTAL COLONY - WEST MAMBALAM 7.30 SRINIVASA THEATRE BUS STOP 7.35 ARANGANATHAR SUBWAY BUS STOP 7.35 KAVERY NAGAR BUS STOP BUS STOP 7.35 C.I.T NAGAR 7.40 SAIDAPET 7.45 SAIDAPET ARCH BUS STOP 7.45 CHINNAMALAI COURT 7.45 VELACHERY 200 FEET ROAD (ERIKARAI) 7.50 VELACHERY 200 FEET ROAD WATER TANK 7.50 COLLEGE ROUTE NO 4 (THIRUVOTRIYUR) BUS STOP TIMING THIRUVOTRIYUR BUS STOP (AJAX) 6.30 THERADI BUS STOP 6.30 ELLAI AMMAN KOIL (JUNCTION) 6.30 RAJA SHOP BUS STOP 6.35 THANGAL BUS STOP 6.40 CROSS ROAD BUS STOP THONDAIRPET 6.40 -

Tamil Nadu Sustainable Urban Development Project Final EA for SWD to Selected Areas of Coc
Tamil Nadu Sustainable Urban Development Project Final EA for SWD to selected areas of CoC Public Disclosure Authorized Tamil Nadu Sustainable Urban Development Project (TNSUDP) Storm Water Drainage Project for the Selected Areas of Chennai Corporation Public Disclosure Authorized Tamil Nadu Sustainable Urban Development Project Public Disclosure Authorized Final Environmental Assessment Report Public Disclosure Authorized January 2015 Storm Water Drains Department, Corporation of Chennai 1 Tamil Nadu Sustainable Urban Development Project Final EA for SWD to selected areas of CoC Executive Summary 1. INTRODUCTION 1. Government of Tamil Nadu has proposed to implement the World Bank supported Tamil Nadu Sustainable Urban Development program (TNSUDP) to improve the delivery of urban services. The provision of Storm Water Drains to selected areas of Corporation of Chennai is one of the sub-projects proposed to be implemented by the Corporation of Chennai (CoC). This component is coordinated by Government of Tamil Nadu with Tamil Nadu Urban Infrastructure Financial Services Ltd. (TNUIFSL), Chennai as the fund Manager. This sub project is proposed to be implemented along the roadsides and canals and doesn’t involve any Land Acquisition and required land is owned by the Corporation of Chennai. 2. PROJECT DESCRIPTION 2. The Storm Water Drainage project for the selected areas of Corporation of Chennai proposed to be taken up under the TNSUDP involves construction of storm water drains and canals in the project area. This sub project proposed to be implemented in the 4 Zones of the CoC. The total length of the drains to be constructed is about 270.33 kilometres including the 11.05 km length in the canals. -

Local Bodies of Tamil Nadu Full Report
PREFACE This Report has been prepared for submission to the Governor under Article 151 of the Constitution. 2. This Report sets out the results of audit under the Comptroller and Auditor General of India’s (Duties, Powers and Conditions of Service) Act, 1971, in respect of financial assistance given to urban local bodies. 3. Matters arising from the Finance and Appropriation Accounts for the year 2004-05, together with other points arising out of audit of transactions of the Government of Tamil Nadu are included in a separate volume of the Report (Civil) of 2004-05. 4. The Report containing the observations arising out of audit of Statutory Corporations, Boards and Government Companies and the Report containing such observations on Revenue Receipts are presented separately. 5. The cases mentioned in this Report are among those which came to notice in the course of test check of accounts of local bodies during the year 2004-05, as well as those which had come to notice in earlier years, but could not be dealt with in previous Reports on Government of Tamil Nadu. Matters relating to the period subsequent to March 2005 have also been included, wherever considered necessary. OVERVIEW This Report, dealing with the results of audit of accounts of urban local bodies contains three Performance Reviews and nine Audit Paragraphs. A synopsis of important audit findings is presented in this overview. I Accounts and Finances of Urban Local Bodies There were six municipal corporations and 151 municipalities in Tamil Nadu as on 31 March 2005. The urban population of the State as per 2001 census was 2.75 crore comprising 44 per cent of total State population.