Development of a Semi-Synthetic Medium Supporting Adherent Growth in Coagulase-Negative Staphylococci
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BD™ Baird-Parker Agar
INSTRUCTIONS FOR USE – READY-TO-USE PLATED MEDIA PA-255084.04 Rev.: Sep 2011 BD Baird-Parker Agar INTENDED USE BD Baird-Parker Agar is a moderately selective and differential medium for the isolation and enumeration of Staphylococcus aureus in foods, environmental, and clinical specimens. PRINCIPLES AND EXPLANATION OF THE PROCEDURE Microbiological method. A variety of media is used for the isolation of Staphylococcus aureus, which plays a major role in food-poisoning and in human clinical infections. The formulation of the present Baird-Parker Agar was published in 1962.1 It is a partially selective medium which applies the ability of staphylococci to reduce tellurite to tellurium and to detect lecithinase from egg lecithin. Baird- Parker Agar is widely used and is included in many standard procedures for testing foods, cosmetics, or swimming pool waters for the presence of Staphylococcus aureus.2-6 It may also be used for the isolation of S. aureus from clinical specimens and is also called Egg- Tellurite-Glycine-Pyruvate Agar (ETGPA).7,8 BD Baird-Parker Agar contains the carbon and nitrogen sources necessary for growth. Glycine, lithium chloride and potassium tellurite act as selective agents. Egg yolk is the substrate to detect lecithinase production, and, in addition, lipase activity. Staphylococci produce dark gray to black colonies due to tellurite reduction; staphylococci that produce lecithinase break down the egg yolk and cause clear zones around respective colonies. An opaque zone of precipitation may form due to lipase activity. The medium must not be used for the isolation of staphylococci other than S. aureus. -

Update on Coagulase-Negative Staphylococci—What the Clinician Should Know
microorganisms Review Update on Coagulase-Negative Staphylococci—What the Clinician Should Know Ricarda Michels †, Katharina Last † , Sören L. Becker and Cihan Papan * Institute of Medical Microbiology and Hygiene, Saarland University, 66424 Homburg, Germany; [email protected] (R.M.); [email protected] (K.L.); [email protected] (S.L.B.) * Correspondence: [email protected]; Tel.: +49-6841-16-23943 † These authors contributed equally to this work. Abstract: Coagulase-negative staphylococci (CoNS) are among the most frequently recovered bacteria in routine clinical care. Their incidence has steadily increased over the past decades in parallel to the advancement in medicine, especially in regard to the utilization of foreign body devices. Many new species have been described within the past years, while clinical information to most of those species is still sparse. In addition, interspecies differences that render some species more virulent than others have to be taken into account. The distinct populations in which CoNS infections play a prominent role are preterm neonates, patients with implanted medical devices, immunodeficient patients, and those with other relevant comorbidities. Due to the property of CoNS to colonize the human skin, contamination of blood cultures or other samples occurs frequently. Hence, the main diagnostic hurdle is to correctly identify the cases in which CoNS are causative agents rather than contaminants. However, neither phenotypic nor genetic tools have been able to provide a satisfying solution to this problem. Another dilemma of CoNS in clinical practice pertains to their extensive Citation: Michels, R.; Last, K.; Becker, antimicrobial resistance profile, especially in healthcare settings. Therefore, true infections caused by S.L.; Papan, C. -

74226 Coagulase Test (Tubes)
74226 Coagulase Test (Tubes) For the detection of coagulase-negative or -positive organisms (Staphylococcus aureus). Product Description: 1 box contains 6 vials (each with 3 ml lyophilized rabbit plasma with EDTA) Store below 8°C and use before expiry date. The rehydrated plasma is stable for 30 days at – 20°C. Directions: a) Conduct the coagulase test on 5 typical and/or 5 atypical colonies on Baird-Paker Agar (Cat. No. 11705) or 5 suspect colonies from other culture media (Staphylococcus Agar, Cat. No. 70193, Blood Agar (Base), Cat. No. 70133, Vogel Johnson Agar, Cat. No. 70195). b) Transfer each of the selected colonies with a sterile inoculation loop to separate culture tubes containing Brain Heart Broth (Cat. No. 53286) and incubate at 37°C for 20-24 hours. c) Rehydrate the lyophilized rabbit plasma with EDTA in 3 ml of distilled water. d) Pipette 0.3 ml of the rabbit plasma into a sterile culture tube using a sterile pipette. e) Carefully mix 0.1 ml of the Brain Heart Broth culture or 1/2 an inoculation loop of colony material from Baird-Paker, Staphylococcus or Blood Agar with the plasma in the sterile culture tube and incubate at 37°C. (Material from Vogel Johnson or Mannitol Salt Phenol Red Agar is not suitable for the test. A Brain Heart Broth culture is favored.) f) Check the tubes every hour for coagulation by gently tipping to the side (do not shake). g) The coagulase test is positive if more than 75% of the tube contents has formed a coherent clot. -

Diagnosis and Microecological Characteristics of Aerobic Vaginitis in Outpatients Based on Preformed Enzymes
Taiwanese Journal of Obstetrics & Gynecology 55 (2016) 40e44 Contents lists available at ScienceDirect Taiwanese Journal of Obstetrics & Gynecology journal homepage: www.tjog-online.com Original Article Diagnosis and microecological characteristics of aerobic vaginitis in outpatients based on preformed enzymes * Zhi-liang Wang a, Lan-yong Fu b, Zheng-ai Xiong a, , Qin Qin a, Teng-hua Yu c, Yu-tong Wu a, Yuan-yuan Hua a, Yong-hong Zhang a a Department of Obstetrics and Gynecology, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China b Department of Obstetrics and Gynecology, Ningde Hospital, Fujian Medical University, Dongqiao District, Ningde City, China c Department of Endocrine and Breast Surgery, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China article info abstract Article history: Objective: Aerobic vaginitis (AV) is a recently proposed term for genital tract infection in women. The Accepted 3 June 2015 diagnosis of AV is mainly based on descriptive diagnostic criteria proposed by Donders and co-workers. The objective of this study is to report AV prevalence in southwest China using an objective assay kit Keywords: based on preformed enzymes and also to determine its characteristics. aerobic vaginitis Materials and methods: A total of 1948 outpatients were enrolled and tested by a commercial diagnostic genital tract infections kit to investigate the AV prevalence and characteristics in southwestern China. The study mainly mixed infection examined the vaginal ecosystem, age distribution, Lactobacillus amount, and changes in pH. Differences vaginal microecological within groups were analyzed by Wilcoxon two-sample test. Results: The AV detection rate is 15.40%. The AV patients were usually seen in the sexually active age group of 20e30 years, followed by those in the age group of 30e40 years. -

Identification and Antibiotic Susceptibility of Coagulase Negative
Br J Ophthalmol 1999;83:771–773 771 Identification and antibiotic susceptibility of coagulase negative staphylococci isolated in Br J Ophthalmol: first published as 10.1136/bjo.83.7.771 on 1 July 1999. Downloaded from corneal/external infections Antonio Pinna, Stefania Zanetti, Mario Sotgiu, Leonardo A Sechi, Giovanni Fadda, Francesco Carta Abstract microbiology laboratory, identification of sta- Aims—To identify and determine anti- phylococci is often limited to a rapid screening biotic susceptibility of coagulase negative test for S aureus, while non-S aureus isolates are staphylococci (CoNS) isolated from pa- simply reported as CoNS species. Apart from tients with chronic blepharitis, purulent being a common component of the normal conjunctivitis, and suppurative keratitis. ocular flora, these bacteria have also been Methods—A retrospective review of all reported to cause chronic blepharitis,23 culture positive cases of chronic blephari- conjunctivitis,45and keratitis.67 tis, purulent conjunctivitis, and suppura- Despite being important ocular pathogens, tive keratitis between July 1995 and CoNS have so far received little attention in December 1996 was performed. Cases in ophthalmology. The purpose of this study was which CoNS were the sole isolates were to identify and determine antibiotic suscepti- analysed. Species identification was per- bility of CoNS isolated from patients with formed by using a commercially available chronic blepharitis, purulent conjunctivitis, standardised biochemical test system. and suppurative keratitis. Antibiotic susceptibility to penicillin, gentamicin, tetracycline, erythromycin, ciprofloxacin, and teicoplanin was deter- Materials and methods mined by agar disc diVusion (Kirby– A retrospective review of all culture positive Bauer method). Teicoplanin resistance cases of chronic blepharitis, purulent conjunc- was confirmed by agar dilution. -

Department of Microbiology Quality Manual Policy # MI GEN Page 1 of 36 Version: 1.5 CURRENT Section: Bacteriology Procedures Su
Policy # MI_GEN Page 1 of 36 Department of Microbiology Quality Manual Version: 1.5 CURRENT Section: Bacteriology Procedures Subject Title: Genital Tract Culture Manual Prepared by QA Committee Issued by: Laboratory Manager Revision Date: 7/14/2021 Approved by Laboratory Director: Next Review Date: 7/14/2023 Microbiologist-in-Chief Uncontrolled When Printed TABLE OF CONTENTS INTRODUCTION ............................................................................................................................... 2 LOWER GENITAL TRACT ............................................................................................................ 4 CERVICAL (ENDOCERVICAL) SWAB .............................................................................. 4 GROUP B STREPTOCOCCUS SCREEN ............................................................................. 7 VAGINITIS SCREEN ............................................................................................................ 10 VAGINAL CULTURE ........................................................................................................... 14 URETHRAL SWAB .............................................................................................................. 18 PENIS SWAB ......................................................................................................................... 20 SEMINAL FLUID .................................................................................................................. 23 UPPER GENITAL TRACT CULTURE ....................................................................................... -

Staphylococci
STAPHYLOCOCCI Staphylococci are typical Gram-positive bacteria forming irregular clusters of cocci. Staphylococci are widespread in nature, although they are mainly found on the skin, skin glands and mucous membranes of mammals and birds, but can cause infection under certain circumstances. 1S. aureus is more pathogenic than the other common members of the genus, S. epidermidis and S. saprophyticus. S. epidermidis has been known to cause various hospital-acquired infections (such as prosthetic or indwelling devices), whereas S. saprophyticus is mainly associated with urinary tract infections in young females who are sexually active. Disease processes with S. aureus are numerous. The portal of entry is variable, since they gain access to the body via the skin, the respiratory tract or the genito- urinary tract. Staphylococcus aureus expresses many potential virulence factors: 1. surface proteins - promote colonization of host tissues 2. leukocidin, kinases, hyaluronidase - invasins that promote bacterial spread in tissues 3. capsule, Protein A - surface factors that inhibit phagocytic engulfment 4. carotenoids, catalase - enhance staphylococcal survival in phagocytes 5. protein A, coagulase - immunological disguises 6. hemolysins, leukotoxin, leukocidin - membrane-damaging toxins that lyse eucaryotic cell membranes 7. 2TSST, 3ET - exotoxins that damage host tissues or otherwise provoke symptoms of disease 8. inherent and acquired resistance to antimicrobial agents. Fig. 1 Virulence determinants of Staphylococcus aureus. 1 S. - Staphylococcus 2 TSST - Toxic Shock Syndrome Toxin 3 ET - Exfoliatin Toxin Staphylococci can cause many forms of infection: 1. S. aureus causes superficial skin lesions (boils) and localized abscesses in other sites. 2. S. aureus causes deep-seated infections, such as osteomyelitis and endocarditis and more serious skin infections (furunculosis). -

Coagulase Activity: Plasma Suitability and Anticoagulants Tech Com: Microbiology
Coagulase Activity: Plasma Suitability and Anticoagulants Tech Com: Microbiology by Cheryl V. Maloney, MT(ASCP) Downloaded from https://academic.oup.com/labmed/article/10/6/358/2640021 by guest on 25 September 2021 Pathogenic Staphylococcus aureus pro anticoagulant tubes were pooled respec duces coagulases that accelerate the tively and each mixed thoroughly. To clotting of plasmas from a number of obtain sterile guinea pig plasma, citrated animal sources. Although coagulase ac guinea pig whole blood (CIBCO Diag tivity correlates with pathogenicity, the nostics, Madison, Wl) was aseptically enzyme itself has a little known role transferred to sterile vials and cen- in the pathogenesis of staphylococcal trifuged. The sterile plasma was then infections. transferred to new sterile vials. Human serum was prepared by collecting blood Several animal plasmas including hu in glass tubes, letting the blood clot man plasma will serve as a satisfactory and pooling several samples. Rabbit substrate for coagulase testing.1 We in plasma (BBL, Cockeysville, MD) was vestigated the nature and action of coagu prepared according to the manufac lase activity by ten isolates of Staphy turer's instructions. lococcus aureus and tested the suitability of four human plasma pools and rabbit To test the sterility of each plasma plasma as substrates. Since guinea pig pool, aliquots were cultured in brain plasma is not suitable for coagulase heart infusion broth (BBL) and observed 2 testing, we studied guinea pig plasma for growth after 24 hours of incubation. to determine if there is a deficiency of coagulase reacting factor (CRF) or if an The tube method of Lenette, Spauld- 3 inhibitor is present. -

Evidence-Based Treatments of Based Treatments of Urinary Tract
2/9/2015 EvidenceEvidence--BasedBased Treatments of Urinary Tract Infections David R. Ellington, MD, FACOG Assistant Professor Division of Urogynecology and Pelvic Reconstructive Surgery Disclosures No Relevant Disclosures Case Study 26 year old G0P0 woman calls your office to report: 3 days of progressive urinary urgency and dysuria She denies fevers, chills, low back pain, or vaginal discharge Two months ago you treated her with a 33--dayday course of Macrobid for presumptive urinary tract infection (UTI), and her symptoms resoldldlved She is otherwise healthy, but this episode would make her 4thth in the past 12 months.. 1 2/9/2015 Objectives Participant will be able to: Describe pertinent history in the evaluation of UTIs Describe the pathophysiology of UTIs Describe diagnostic methods and criteria of various types of UTIsof UTIs Describe techniques, accuracy, sensitivity, and specificity of: dipstick urinalysis, microscopic urinalysis, and urine cultureculture Describe indications for upper tract imaging/cystoscopy Describe evidence for various treatment options Definitions BacteriuriaBacteriuria:: presence of bacteria in urine infection, colonization, contamination Pyyyuria: presence of WBC in urine inflammatory response of urothelium UTI: inflammatory response of the urothelium to bacterial invasion associated with bacteriuria and pyuria Bacteriuria UTI Pyuria/ Inflammation 2 2/9/2015 What if both are NOT present? Bacteriuria without pyuria Colonization Contamination Pyuria without bacteriuria Bladder -
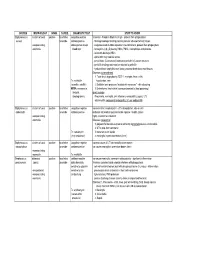
Bacteria Chart1
SPECIES MORPHOLOGY GRAM O2 REQ. DIAGNOSTIC TEST STUFF TO KNOW Staphylococcus clusters of cocci positive facultative coagulase positive Virulence: -Protein A binds Fc of IgG - protects from phagocytosis aureus anaerobe catalase positive -fibrinogen/collagen binding proteins promote adhesion to host tissues -nonsporulating stains gold on sheep -coagulase leads to fibrin deposition around bacteria, protects from phagocytosis -nonmotile blood agar -hemolysins (α,β,γ,δ) destroy RBCs, PMNs, macrophages and platelets -leukocidin destroys WBCs -alpha toxin may mediate sepsis -penicillinase (β-lactamase) inactivates penicillin's β-lactam structure -penicillin binding protein can be resistant to penicillin -hyaluronidase, staphylokinase, lipase, protease break down host tissues Diseases: toxin-mediated 1. Toxic shock by producing TSST-1; myalgias, fever, chills, Tx: methicillin hypotension, rash (oxacillin, nafcillin) 2. Exfoliatin toxin produces "scalded skin syndrome" - skin sloughing MRSA: vancomycin, 3. Enterotoxins (heat stable) cause gastroenteritis (food poisoning) linezolid, direct invasion streptogramins Pneumonia, meningitis, skin infections, endocarditis, sepsis, UTI, osteomyelitis, nosocomial endocarditis, IV user endocarditis Staphylococcus clusters of cocci positive facultative coagulase negative commensal in nasopharynx in ~30% of population, also on skin epidermidis anaerobe catalase positive elaborate extracellular polysaccharide capsule = biofilm (slime) -nonsporulating highly resistant to antibiotics! -nonmotile Diseases: nosocomial -

Management of Vaginal Discharge
VAGINAL DISCHARGE MANAGEMENT OF VAGINAL DISCHARGE Vaginal discharge may be entirely normal, or a sign of infection. In either case, it is a relatively common presentation in general practice and deserves some attention. The normal anatomy, physiology and microbial ecology of the vagina are age- dependent as are the sources of vaginal infections. In the neonatal period, the vagina is influenced by maternal oestrogen and has a stratified squamous epithe- lium. Later, and until puberty, the vagina is lined by cuboidal cells and the pH is around 7.0. Following puberty, oestrogen causes a change to stratified squa- mous epithelium. The predominant microbial flora at this age are lactobacilli such as L. crispatus and L. jensenii, which produce lactic acid, and the pH falls to 4.0 - 4.5. The other vaginal flora include diphtheroids, β-haemolytic strepto- cocci, coliforms and coagulase-negative staphylococci.1 VAGINAL DISCHARGE IN PRE-PUBERTAL GIRLS Vaginal symptoms including a discharge in pre-pubertal girls ought to be investi- A A HOOSEN gated microbiologically as the sexually transmitted (ST) pathogens Neisseria gon- 2 MSc, MB ChB, MMed, FCPath orrhoeae and Chlamydia trachomatis may be isolated. Chief Specialist and Head Other non-ST pathogens include β-haemolytic streptococci Group A (S. pyo- Department of Microbiological Pathology genes), Shigella flexneri and Enterobius vermicularis. MEDUNSA Professor Anwar Hoosen is currently Head of VAGINAL DISCHARGES IN POST-PUBERTAL WOMEN the NHLS’s Microbiology Laboratory for the Vaginal infection is one of the top 25 reasons for women to consult doctors in the Northern Branch. He has conducted numerous USA. -

Distinguishing True Coagulase-Negative Staphylococcus Infections from Contaminants in the Neonatal Intensive Care Unit
Journal of Perinatology (2013) 33,52–58 r 2013 Nature America, Inc. All rights reserved. 0743-8346/13 www.nature.com/jp ORIGINAL ARTICLE Distinguishing true coagulase-negative Staphylococcus infections from contaminants in the neonatal intensive care unit CM Healy1,2, CJ Baker1,2,3, DL Palazzi1,2, JR Campbell1,2 and MS Edwards1,2 1Department of Pediatrics, Baylor College of Medicine, Houston, TX, USA; 2Woman’s Hospital of Texas, Houston, TX, USA and 3Department of Molecular Virology and Microbiology, Baylor College of Medicine, Houston, TX, USA can rapidly progress and even be fatal before the infecting Objective: To characterize true coagulase-negative Staphylococcus (CoNS) pathogen has been identified.3,4,8,9 infections in infants receiving neonatal intensive care. Coagulase-negative staphylococci (CoNS) are commensal skin Study Design: Retrospective cohort study of neonatal intensive care unit flora but these organisms account for up to one-half of reported (NICU) infants with clinical sepsis and CoNS isolated from X2 blood bloodstream infections in very low birth weight (<1500 g) 3,4,7–9,11 cultures (BCs) or one BC and a sterile site (proved infection) or CoNS infants. CoNS present a particular dilemma because their isolated from one BC and deemed significant after blinded data review isolation from a single blood culture (BC) in an NICU patient can (probable infection). reflect contamination rather than true bacteremia. The difficulty inherent in distinguishing true infections from culture Result: In all, 98% of 40 proved and 96% of 55 probable infections contaminants potentially results in over-representation of CoNS in occurred in infants with birth weight (BW) <2000 g and gestation <34 NICU sepsis incidence data, despite attempts to define infection weeks.