Better Health. Within Reach. Every Day
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

In the United States District Court for the District Of
IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE FOREST LABORATORIES, LLC and ) FOREST LABORATORIES ) HOLDINGS, LTD., ) ) Plaintiffs, ) ) v. ) Civ.No.14-1119-SLR ) SIGMAPHARM LABORATORIES, LLC, ) et al., ) ) Defendants. ) Jack B. Blumenfeld, Esquire and Maryellen Noreika, Esquire of Morris, Nichols, Arsht & Tunnell LLP, Wilmington, Delaware. Counsel for Plaintiffs. Of Counsel: Howard W. Levine, Esquire, Sanya Sukduang, Esquire, Jonathan R. Davies, Esquire, Courtney B. Gasp, Esquire, and Charles E. Lipsey, Esquire of Finnegan, Henderson, Farabow, Garrett & Dunner LLP. John C. Phillips, Esquire, David A. Bilson, Esquire and Megan C. Haney of Phillips, Goldman, Mclaughlin & Hall, P.A., Wilmington, Delaware. Counsel for Defendant Sigmapharm Laboratories, LLC. Of Counsel: Anthony G. Simon, Esquire, Anthony R. Friedman, Esquire, Benjamin R. Askew, Esquire, and Michael P. Kella, Esquire of The Simon Law Firm, P.C. Karen Elizabeth Keller, Esquire and Jeffrey Thomas Castellano, Esquire of Shaw Keller, LLP, Wilmington, Delaware. Counsel for Defendants Hikma Pharmaceuticals LLC, Hikma Pharmaceuticals PLC, and West-Ward Pharmaceutical Corp. Of Counsel: lmron T. Aly, Esquire, Joel M. Wallace, Esquire, and Helen H. Ji, Esquire of Schiff Hardin LLP. Richard D. Kirk, Esquire, Stephen B. Brauerman, Esquire and Sara E. Bussiere, Esquire of Bayard, P.A., Wilmington, Delaware. Counsel for Defendant Breckenridge Pharmaceutical, Inc. Of Counsel: Beth D. Jacob, Esquire, Clifford Katz, Esquire, and Malavika A. Rao, Esquire of Kelley, Drye & Warren LLP. Karen Pascale, Esquire and Pilar G. Kraman, Esquire of Young, Conaway, Stargatt & Taylor, LLP, Wilmington, Delaware. Counsel for Defendants Alembic Pharmaceuticals Ltd., Alembic Global Holding SA and Alembic Pharmaceuticals, Inc. Of Counsel: Steven J. Lee, Esquire, Michael K. -

Preclinical Development & IND Filing: Nuts, Bolts, Best Practices
“Preclinical Development & IND Filing: Nuts, Bolts, Best Practices and Regulatory Aspects.” Speakers: Amit Kalgutkar (Pfizer), Chandra Prakash (Agios), Sanjeev Thohan (Novartis), Li- Chun Wang (Takeda), Wei Yin (Biogen) Organizers: Sanjeev Thohan and Chandra Prakash Date: 6/29/2017 Time: 8:30 am – 5.00 pm Location: Boston/Cambridge Area: Marriott Kendall Square, 50 Broadway, Cambridge MA 02142 Fees: $175 - Regular (before June 1st, 2017), $225 - Regular (after June 1st, 2017); $125 - Unemployed & Academic; $2000 - Major Sponsorship; $475 - Vendor Show Registration: www.PBSS.org Workshop Description: An investigational new drug application (IND) is an important milestone that marks the entry of a molecule into clinical development. Knowing the objectives, expectations and processes of assembling an IND is a key to not only successful filing, but also a promising clinical development path forward. Often there are cases where too many of our “nice-to-have” studies crowd the package at the expense of critical study needs/issues. This can lead to significant delays in clinical developments with back-and-forth of Q&A sessions both internally and with regulatory agencies. As we have seen, the regulatory landscape is changing as rapidly as the industry innovates into new therapeutic modalities. Therefore, it is critical to keep up to date on regulatory requirements and the industry’s best practices in different aspects of the IND: non- clinical safety, PK, CMC, and clinical plans. In this workshop, our speakers who bring years of experience with multiple successful IND filings, will discuss systematically the preclinical studies required for small molecule IND’s as well as the nuts and bolts of putting together a high–quality IND package. -

Assessment Report COVID-19 Vaccine Astrazeneca EMA/94907/2021
29 January 2021 EMA/94907/2021 Committee for Medicinal Products for Human Use (CHMP) Assessment report COVID-19 Vaccine AstraZeneca Common name: COVID-19 Vaccine (ChAdOx1-S [recombinant]) Procedure No. EMEA/H/C/005675/0000 Note Assessment report as adopted by the CHMP with all information of a commercially confidential nature deleted. Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000 An agency of the European Union © European Medicines Agency, 2021. Reproduction is authorised provided the source is acknowledged. Table of contents 1. Background information on the procedure .............................................. 7 1.1. Submission of the dossier ..................................................................................... 7 1.2. Steps taken for the assessment of the product ........................................................ 9 2. Scientific discussion .............................................................................. 12 2.1. Problem statement ............................................................................................. 12 2.1.1. Disease or condition ........................................................................................ 12 2.1.2. Epidemiology and risk factors ........................................................................... 12 2.1.3. Aetiology and pathogenesis ............................................................................. -

Factset-Top Ten-0521.Xlsm
Pax International Sustainable Economy Fund USD 7/31/2021 Port. Ending Market Value Portfolio Weight ASML Holding NV 34,391,879.94 4.3 Roche Holding Ltd 28,162,840.25 3.5 Novo Nordisk A/S Class B 17,719,993.74 2.2 SAP SE 17,154,858.23 2.1 AstraZeneca PLC 15,759,939.73 2.0 Unilever PLC 13,234,315.16 1.7 Commonwealth Bank of Australia 13,046,820.57 1.6 L'Oreal SA 10,415,009.32 1.3 Schneider Electric SE 10,269,506.68 1.3 GlaxoSmithKline plc 9,942,271.59 1.2 Allianz SE 9,890,811.85 1.2 Hong Kong Exchanges & Clearing Ltd. 9,477,680.83 1.2 Lonza Group AG 9,369,993.95 1.2 RELX PLC 9,269,729.12 1.2 BNP Paribas SA Class A 8,824,299.39 1.1 Takeda Pharmaceutical Co. Ltd. 8,557,780.88 1.1 Air Liquide SA 8,445,618.28 1.1 KDDI Corporation 7,560,223.63 0.9 Recruit Holdings Co., Ltd. 7,424,282.72 0.9 HOYA CORPORATION 7,295,471.27 0.9 ABB Ltd. 7,293,350.84 0.9 BASF SE 7,257,816.71 0.9 Tokyo Electron Ltd. 7,049,583.59 0.9 Munich Reinsurance Company 7,019,776.96 0.9 ASSA ABLOY AB Class B 6,982,707.69 0.9 Vestas Wind Systems A/S 6,965,518.08 0.9 Merck KGaA 6,868,081.50 0.9 Iberdrola SA 6,581,084.07 0.8 Compagnie Generale des Etablissements Michelin SCA 6,555,056.14 0.8 Straumann Holding AG 6,480,282.66 0.8 Atlas Copco AB Class B 6,194,910.19 0.8 Deutsche Boerse AG 6,186,305.10 0.8 UPM-Kymmene Oyj 5,956,283.07 0.7 Deutsche Post AG 5,851,177.11 0.7 Enel SpA 5,808,234.13 0.7 AXA SA 5,790,969.55 0.7 Nintendo Co., Ltd. -
Astrazeneca-Oxford Vaccine Approved for Use in the U.K
P2JW366000-6-A00100-17FFFF5178F ****** THURSDAY,DECEMBER 31,2020~VOL. CCLXXVI NO.154 WSJ.com HHHH $4.00 DJIA 30409.56 À 73.89 0.2% NASDAQ 12870.00 À 0.2% STOXX 600 400.25 g 0.3% 10-YR. TREAS. À 3/32 , yield 0.926% OIL $48.40 À $0.40 GOLD $1,891.00 À $10.50 EURO $1.2300 YEN 103.21 Deadly Attack at Airport Targets New Yemen Government U.S. IPO What’s News Market Reaches Business&Finance Record nvestorspiled into IPOs Iat a record rate in 2020, with companies raising Total $167.2 billion via 454 of- ferings on U.S. exchanges this year through Dec. 24. Few see signs of letup Few expect the euphoria after companies raise to wear off soon. A1 more than $167 billion Detenteisending in the global fight over tech taxes, despite pandemic with Franceresuming collec- tion of itsdigital-services tax BY MAUREEN FARRELL and the U.S. poised to retali- atewith tariffs.Other coun- Defying expectations,inves- tries areset to join the fray. A1 S tors piled intoinitial public of- China finished 2020 PRES feringsatarecordrateiN with a 10th consecutive TED 2020, and few expect the eu- month of expansion in its CIA phoria to wear off soon. manufacturing sector. A7 SO Companies raised $167.2 AS TheEUand China agreed TENSIONS HIGH: People fled after an explosion Wednesday at the airport in Aden, Yemen, moments after members of the billion through 454 offerings in principle on an invest- country’s newly sworn-in cabinet arrived. At least 22 people were killed, but all the members of the cabinet were safe. -

United States District Court for the District of Columbia
Case 1:12-cv-00472-BAH Document 42 Filed 07/05/12 Page 1 of 49 UNITED STATES DISTRICT COURT FOR THE DISTRICT OF COLUMBIA ASTRAZENECA PHARMACEUTICALS LP, Plaintiff, v. Civil Action No. 12-00472 (BAH) Judge Beryl A. Howell FOOD AND DRUG ADMINISTRATION, et al., Defendants. AMENDED MEMORANDUM OPINION Plaintiff AstraZeneca Pharmaceuticals LP (“AstraZeneca”) has manufactured the drug quetiapine fumarate (“quetiapine”) under the brand name Seroquel® (“Seroquel”) since 1997 without generic competition. AstraZeneca brought this lawsuit, which presents a question of statutory interpretation, against the Food and Drug Administration, Margaret A. Hamburg, M.D., Commissioner of Food and Drugs, and Kathleen Sebelius, Secretary of Health and Human Services (collectively, “the FDA”), to challenge the FDA’s approval, on March 27, 2012, of generic versions of Seroquel. See Complaint, ECF No. 1 (“Compl.”), ¶ 3. AstraZeneca believes that, under the plain language of 21 U.S.C. § 355(j)(5)(F)(iv), codifying Section 505(j)(5)(F)(iv) of the Federal Food, Drug, and Cosmetic Act (“FDCA”), it is entitled to total market exclusivity until December 2, 2012 for the safety information encapsulated in “Table 2,” which was approved for all Seroquel labels on December 2, 2009 and must be included on the labels of all generic versions of quetiapine. Based upon this belief, AstraZeneca seeks a judgment that the FDA’s recent approval of generic versions of quetiapine, while AstraZeneca retains exclusivity over Table 2, violated AstraZeneca’s exclusivity rights and was arbitrary, capricious, and contrary to law. 1 Case 1:12-cv-00472-BAH Document 42 Filed 07/05/12 Page 2 of 49 Pending before the Court are Cross-Motions for Summary Judgment filed by AstraZeneca, ECF No. -

Cephalon Provigil
r* jfS* 1 ll JS 44 {Rev. 07/16) SHEET The JS 44 civil cover sheet and the information contained herein neither replace nor supplement the filing and service of pleadings or other papers as required by law, except as provided by local rules of court. This form, approved by the Judicial Conference of the United States in September 1974, is required for the use of the Clerk of Court for the purpose ofinitiating the civil docket sheet. (SEE INSTRUCTIONS ON NEXT PAGE OF THIS FORM.) 1. (a) PLAINTIFFS DEFENDANTS State of New York, et al. Cephalon, Inc. et al. (b) County of Residence of First Listed Plaintiff County of Residence of First Listed Defendant (EXCEPT IN U.S. PLAINTIFF CASES) (IN U.S. PLAINTIFF CASES ONLY) NOTE: IN LAND CONDEMNATION CASES, USE THE LOCATION OF THE TRACT OF LAND INVOLVED. (c) Attorneys (Firm Name, Address, and Telephone Number) Attorneys (If Known) Office of the Attorney General, Commonwealth of Pennsylvania, 14® Floor Jay Lefkowitz, Kirkland & Ellis, 601 Lexington Avenue, New York, NY 10022 Strawberry Square, Harrisburg, PA 17120(717)787-4530 (212)446-4800 II. BASIS OF JURISDICTION (Placean "X" in One Box Only) III. CITIZENSHIP OF PRINCIPAL PARTIES (Placean "X" inOne Box for Plaintiff (For Diversify Cases Only) and One Box for Defendant) • 1 U.S. Government ^ 3 Federal Question PTF DEF PTF DEF Plaintiff (U.S. Government Not a Party) Citizen of This State Q 1 • 1 Incorporated or Principal Place Q 4 HI 4 of Business In This State l~l 2 U.S. Government • 4 Diversity Citizen of Another State • 2 Q 2 Incorporated and Principal Place G 5 Q 5 Defendant (Indicate Citizenship of Parties in Item III) of Business In Another State Citizen or Subject of a Q3 D 3 Foreign Nation D 6 06 Foreign Country IV. -

Annex 1: Parker Review Survey Results As at 2 November 2020
Annex 1: Parker Review survey results as at 2 November 2020 The data included in this table is a representation of the survey results as at 2 November 2020, which were self-declared by the FTSE 100 companies. As at March 2021, a further seven FTSE 100 companies have appointed directors from a minority ethnic group, effective in the early months of this year. These companies have been identified through an * in the table below. 3 3 4 4 2 2 Company Company 1 1 (source: BoardEx) Met Not Met Did Not Submit Data Respond Not Did Met Not Met Did Not Submit Data Respond Not Did 1 Admiral Group PLC a 27 Hargreaves Lansdown PLC a 2 Anglo American PLC a 28 Hikma Pharmaceuticals PLC a 3 Antofagasta PLC a 29 HSBC Holdings PLC a InterContinental Hotels 30 a 4 AstraZeneca PLC a Group PLC 5 Avast PLC a 31 Intermediate Capital Group PLC a 6 Aveva PLC a 32 Intertek Group PLC a 7 B&M European Value Retail S.A. a 33 J Sainsbury PLC a 8 Barclays PLC a 34 Johnson Matthey PLC a 9 Barratt Developments PLC a 35 Kingfisher PLC a 10 Berkeley Group Holdings PLC a 36 Legal & General Group PLC a 11 BHP Group PLC a 37 Lloyds Banking Group PLC a 12 BP PLC a 38 Melrose Industries PLC a 13 British American Tobacco PLC a 39 Mondi PLC a 14 British Land Company PLC a 40 National Grid PLC a 15 BT Group PLC a 41 NatWest Group PLC a 16 Bunzl PLC a 42 Ocado Group PLC a 17 Burberry Group PLC a 43 Pearson PLC a 18 Coca-Cola HBC AG a 44 Pennon Group PLC a 19 Compass Group PLC a 45 Phoenix Group Holdings PLC a 20 Diageo PLC a 46 Polymetal International PLC a 21 Experian PLC a 47 -

Constituents & Weights
2 FTSE Russell Publications 19 August 2021 FTSE 100 Indicative Index Weight Data as at Closing on 30 June 2021 Index weight Index weight Index weight Constituent Country Constituent Country Constituent Country (%) (%) (%) 3i Group 0.59 UNITED GlaxoSmithKline 3.7 UNITED RELX 1.88 UNITED KINGDOM KINGDOM KINGDOM Admiral Group 0.35 UNITED Glencore 1.97 UNITED Rentokil Initial 0.49 UNITED KINGDOM KINGDOM KINGDOM Anglo American 1.86 UNITED Halma 0.54 UNITED Rightmove 0.29 UNITED KINGDOM KINGDOM KINGDOM Antofagasta 0.26 UNITED Hargreaves Lansdown 0.32 UNITED Rio Tinto 3.41 UNITED KINGDOM KINGDOM KINGDOM Ashtead Group 1.26 UNITED Hikma Pharmaceuticals 0.22 UNITED Rolls-Royce Holdings 0.39 UNITED KINGDOM KINGDOM KINGDOM Associated British Foods 0.41 UNITED HSBC Hldgs 4.5 UNITED Royal Dutch Shell A 3.13 UNITED KINGDOM KINGDOM KINGDOM AstraZeneca 6.02 UNITED Imperial Brands 0.77 UNITED Royal Dutch Shell B 2.74 UNITED KINGDOM KINGDOM KINGDOM Auto Trader Group 0.32 UNITED Informa 0.4 UNITED Royal Mail 0.28 UNITED KINGDOM KINGDOM KINGDOM Avast 0.14 UNITED InterContinental Hotels Group 0.46 UNITED Sage Group 0.39 UNITED KINGDOM KINGDOM KINGDOM Aveva Group 0.23 UNITED Intermediate Capital Group 0.31 UNITED Sainsbury (J) 0.24 UNITED KINGDOM KINGDOM KINGDOM Aviva 0.84 UNITED International Consolidated Airlines 0.34 UNITED Schroders 0.21 UNITED KINGDOM Group KINGDOM KINGDOM B&M European Value Retail 0.27 UNITED Intertek Group 0.47 UNITED Scottish Mortgage Inv Tst 1 UNITED KINGDOM KINGDOM KINGDOM BAE Systems 0.89 UNITED ITV 0.25 UNITED Segro 0.69 UNITED KINGDOM -
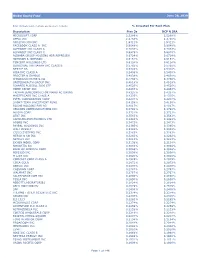
Global Equity Fund Description Plan 3S DCP & JRA MICROSOFT CORP
Global Equity Fund June 30, 2020 Note: Numbers may not always add up due to rounding. % Invested For Each Plan Description Plan 3s DCP & JRA MICROSOFT CORP 2.5289% 2.5289% APPLE INC 2.4756% 2.4756% AMAZON COM INC 1.9411% 1.9411% FACEBOOK CLASS A INC 0.9048% 0.9048% ALPHABET INC CLASS A 0.7033% 0.7033% ALPHABET INC CLASS C 0.6978% 0.6978% ALIBABA GROUP HOLDING ADR REPRESEN 0.6724% 0.6724% JOHNSON & JOHNSON 0.6151% 0.6151% TENCENT HOLDINGS LTD 0.6124% 0.6124% BERKSHIRE HATHAWAY INC CLASS B 0.5765% 0.5765% NESTLE SA 0.5428% 0.5428% VISA INC CLASS A 0.5408% 0.5408% PROCTER & GAMBLE 0.4838% 0.4838% JPMORGAN CHASE & CO 0.4730% 0.4730% UNITEDHEALTH GROUP INC 0.4619% 0.4619% ISHARES RUSSELL 3000 ETF 0.4525% 0.4525% HOME DEPOT INC 0.4463% 0.4463% TAIWAN SEMICONDUCTOR MANUFACTURING 0.4337% 0.4337% MASTERCARD INC CLASS A 0.4325% 0.4325% INTEL CORPORATION CORP 0.4207% 0.4207% SHORT-TERM INVESTMENT FUND 0.4158% 0.4158% ROCHE HOLDING PAR AG 0.4017% 0.4017% VERIZON COMMUNICATIONS INC 0.3792% 0.3792% NVIDIA CORP 0.3721% 0.3721% AT&T INC 0.3583% 0.3583% SAMSUNG ELECTRONICS LTD 0.3483% 0.3483% ADOBE INC 0.3473% 0.3473% PAYPAL HOLDINGS INC 0.3395% 0.3395% WALT DISNEY 0.3342% 0.3342% CISCO SYSTEMS INC 0.3283% 0.3283% MERCK & CO INC 0.3242% 0.3242% NETFLIX INC 0.3213% 0.3213% EXXON MOBIL CORP 0.3138% 0.3138% NOVARTIS AG 0.3084% 0.3084% BANK OF AMERICA CORP 0.3046% 0.3046% PEPSICO INC 0.3036% 0.3036% PFIZER INC 0.3020% 0.3020% COMCAST CORP CLASS A 0.2929% 0.2929% COCA-COLA 0.2872% 0.2872% ABBVIE INC 0.2870% 0.2870% CHEVRON CORP 0.2767% 0.2767% WALMART INC 0.2767% -

Global Focus – Economic Outlook Q2-2021
Global Research Global Focus – Economic Outlook Q2-2021 Global growth – An uneven race research.sc.com Standard Chartered Global Research is available across all iOS and Android devices. Our intuitive, accessible and customisable apps* allow you to receive our reports, forecasts, audio-visual presentations and interactive data visualisation tools on-the-go. * Click the icons to download or search ‘Standard Chartered Global Research’ in the app store. If you are in scope for MiFID II and want to opt out of our Research services, please contact us. Issuer of Report Standard Chartered Bank Important disclosures and analyst certifications can be found in the Disclosures Appendix All rights reserved. Standard Chartered Bank 2021 https://research.sc.com Global Focus – Economic Outlook Q2-2021 Table of contents Global overview 3 Cameroon – Moving towards recovery 75 Global growth – An uneven race 4 Côte d’Ivoire – A series of unfortunate events 76 Where we differ from consensus 10 Ethiopia – A particularly difficult quarter ahead 77 Global charts 12 Gabon – The oil price reprieve 78 Ghana – The long reach of the COVID crisis 79 Geopolitical economics 14 Kenya – Third-wave woes 80 Biden’s democracy club 15 Mozambique – Delayed but not denied 81 Economies – Asia 20 Nigeria – Higher oil, faltering reform 82 Asia – Top charts 21 Senegal – Flaring tensions 83 Asia – Macro trackers 22 South Africa – Bracing for a third wave 84 Australia – Chugging along 24 Tanzania – Transition 86 Bangladesh – Turning around 26 Uganda – Oil to drive growth acceleration -

FDA Listing of Authorized Generics As of July 1, 2021
FDA Listing of Authorized Generics as of July 1, 2021 Note: This list of authorized generic drugs (AGs) was created from a manual review of FDA’s database of annual reports submitted to the FDA since January 1, 1999 by sponsors of new drug applications (NDAs). Because the annual reports seldom indicate the date an AG initially entered the market, the column headed “Date Authorized Generic Entered Market” reflects the period covered by the annual report in which the AG was first reported. Subsequent marketing dates by the same firm or other firms are not included in this listing. As noted, in many cases FDA does not have information on whether the AG is still marketed and, if not still marketed, the date marketing ceased. Although attempts have been made to ensure that this list is as accurate as possible, given the volume of d ata reviewed and the possibility of database limitations or errors, users of this list are cautioned to independently verify the information on the list. We welcome comments on and suggested changes (e.g., additions and deletions) to the list, but the list may include only information that is included in an annual report. Please send suggested changes to the list, along with any supporting documentation to: [email protected] A B C D E F G H I J K L M N O P Q R S T U V X Y Z NDA Applicant Date Authorized Generic Proprietary Name Dosage Form Strength Name Entered the Market 1 ACANYA Gel 1.2% / 2.5% Bausch Health 07/2018 Americas, Inc.