Structural Plumage Colour As a Signal of Mate Quality in Tree Swallows
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Barn Swallows AKA: Mud Swallows
Barn Swallows AKA: Mud Swallows. Close relatives: Purple Martin, Cliff Swallow, Tree Swallow Scientific Classification: Animalia, Chordata, Aves, Passeriformes, Hirundinidae; Hirundo; H. rustica. Bird Size & Markings: Adult Barn Swallows are about 7” long, stand 4” high and have a 13” wingspan. They weigh less than 1 ounce. Males have metallic blue back, wings, and tail with rufous to tawny underside. The blue crown and face is contrasted with the cinnamon forehead and throat. Females are not as brightly colored. Habitat: You can find Barn Swallows feeding in open habitats such as fields, parks, marshes, meadows, ponds, and coastal waters. Their nests are often easy to spot under protected overhangs. Nesting/Dens: Barn Swallows lay 3 to 7 eggs in each brood and can hatch broods twice a year. Brood fledge in about 2 weeks. Both sexes construct the nest of mud pel- lets. If attached to a wall or beam, the nest is half-cup shaped. If on top of a surface, A mating pair of Barn Swallows. They prefer to the nest forms a perfect small cup about 3” wide. Nest sites are almost exclusively at- build their nests where there is overhead pro- tached to man made structures with overhead protection; roof eaves, the underside tection from the weather. of bridges, inside barns and stables, etc. Food: Barn Swallows eat insects - both flying and terrestrial. They usually take rela- tively large, single insects rather than feeding on swarms of smaller prey. They typi- cally feed just above shallow waters or turf. They have been known to follow tractors and livestock, eating the insects that are flushed out by their movement. -

The Evolution of Nest Construction in Swallows (Hirundinidae) Is Associated with the Decrease of Clutch Size
© Biologiezentrum Linz/Austria; download unter www.biologiezentrum.at Linzer biol. Beitr. 38/1 711-716 21.7.2006 The evolution of nest construction in swallows (Hirundinidae) is associated with the decrease of clutch size P. HENEBERG A b s t r a c t : Variability of the nest construction in swallows (Hirundinidae) is more diverse than in other families of oscine birds. I compared the nest-building behaviour with pooled data of clutch size and overall hatching success for 20 species of swallows. The clutch size was significantly higher in temperate cavity-adopting swallow species than in species using other nesting modes including species breeding in evolutionarily advanced mud nests (P<0.05) except of the burrow-excavating Bank Swallow. Decrease of the clutch size during the evolution of nest construction is not compensated by the increase of the overall hatching success. K e y w o r d s : Hirundinidae, nest construction, clutch size, evolution Birds use distinct methods to avoid nest-predation: active nest defence, nest camouflage and concealment or sheltered nesting. While large and powerful species prefer active nest-defence, swallows and martins usually prefer construction of sheltered nests (LLOYD 2004). The nests of swallows vary from natural cavities in trees and rocks, to self-exca- vated burrows to mud retorts and cups attached to vertical faces. Much attention has been devoted to the importance of controlling for phylogeny in com- parative tests (HARVEY & PAGEL 1991), including molecular phylogenetic studies of swallows (WINKLER & SHELDON 1993). Interactions between the nest-construction va- riability and the clutch size, however, had been ignored. -

Top 10 Reasons Why People Fail to Attract Purple Martins
Ten Reasons Why People Fail to Attract Purple Martins Over one million North Americans maintain housing for way out in open fields, or next to isolated ponds, rarely lished sites, martins will not recognize, as potential Purple Martins. Unfortunately, only a small percentage of results in successful martin attraction. Martin housing breeding sites, any martin housing with closed holes. these folks successfully attract breeding martins. Below is should be placed in the center of the most open spot A few compartments should be left open on each side a list of the top ten reasons why so many people fail. Your available, about 30’-100’ from human housing. If your of the house. chances of attracting martins will greatly increase if you martin housing hasn’t attracted nesting martins and In contrast, landlords that had breeding martins avoid making these common mistakes. isn’t placed within 100 feet of your house, try moving the previous year can leave their housing completely it closer. closed up, if they choose, until the martins return and 1. Housing placed too close to tall trees or in land on the housing. They can do this because Purple yards that are too enclosed. The main reason people 4. Housing not painted white. Although martins Martins exhibit a very high level of site fidelity — once fail to attract martins is that they place their martin have been known to nest in houses and gourds painted they have bred successfully at a specific location, the housing incorrectly within their yards, or their yards are other colors, white housing seems to attract them best. -

Tree Swallow in Scilly: New to the Western Palearctic Jeremy Hickman
Tree Swallow in Scilly: new to the Western Palearctic Jeremy Hickman The Isles of Scilly is renowned as a haven for displaced migrant birds, and the autumn pilgrimage of observers in September and October is famous in ornithological circles. June is usually a quiet month for numbers of visiting birdwatchers, as are the other months outside the autumn, but June 1990 was the exception. In one five-day period, between 800 and 1,000 people came to see one bird: the first record for Britain & Ireland, Europe and the Western Palearctic of a North American species, Tree Swallow Tachycineta bicolor. On Wednesday 6th June 1990, having finished my shift behind the bar in the Mermaid Inn, I decided to go to Porth Hellick. I watched from the main hide for a while and could hardly believe how devoid of bird life it was. I could not even console myself by counting the Moorhens Gallinula chloropus. At about 19.00 BST, five hirundines approached low over the pool: one House Martin Delichon urbica, three Barn Swallows Hirundo rustica and another bird. This fifth bird gave the impression of a martin, but with no white rump and a glossy blue-green mantle and crown, and pure white underparts. My heart sank as the bird then flew to the back of the pool and began hawking around the pines and surrounding fields. I rushed to Sluice to obtain closer views and to note its plumage in detail. It appeared slightly bigger and bulkier in the body than a House Martin, with broader-based wings and more powerful flight. -

The First Mangrove Swallow Recorded in the United States
The First Mangrove Swallow recorded in the United States INTRODUCTION tem with a one-lane unsurfaced road on top, Paul W. Sykes, Jr. The Space Coast Birding and Wildlife Festival make up the wetland part of the facility (Fig- USGS Patuxent Wildlife Research Center was held at Titusville, Brevard County, ures 1 and 2). The impoundments comprise a Florida on 13–17 November 2002. During total of 57 hectares (140 acres), are kept Warnell School of Forest Resources the birding competition on the last day of the flooded much of the time, and present an festival, the Canadian Team reported seeing open expanse of shallow water in an other- The University of Georgia several distant swallows at Brevard County’s wise xeric landscape. Patches of emergent South Central Regional Wastewater Treat- freshwater vegetation form mosaics across Athens, Georgia 30602-2152 ment Facility known as Viera Wetlands. open water within each impoundment and in They thought these were either Cliff the shallows along the dikes. A few trees and (email: [email protected]) (Petrochelidon pyrrhonota) or Cave (P. fulva) aquatic shrubs are scattered across these wet- Swallows. lands. Following his participation at the festival, At about 0830 EST on the 18th, Gardler Gardler looked for the swallows on 18 stopped on the southmost dike of Cell 1 Lyn S. Atherton November. The man-made Viera Wetlands (Figure 2) to observe swallows foraging low are well known for waders, waterfowl, rap- over the water and flying into the strong 1100 Pinellas Bayway, I-3 tors, shorebirds, and open-country passer- north-to-northwest wind. -

Colorado Field Ornithologists the Colorado Field Ornithologists' Quarterly
Journal of the Colorado Field Ornithologists The Colorado Field Ornithologists' Quarterly VOL. 36, NO. 1 Journal of the Colorado Field Ornithologists January 2002 Vol. 36, No. 1 Journal of the Colorado Field Ornithologists January 2002 TABLE OF C ONTENTS A LETTER FROM THE E DITOR..............................................................................................2 2002 CONVENTION IN DURANGO WITH KENN KAUFMANN...................................................3 CFO BOARD MEETING MINUTES: 1 DECEMBER 2001........................................................4 TREE-NESTING HABITAT OF PURPLE MARTINS IN COLORADO.................................................6 Richard T. Reynolds, David P. Kane, and Deborah M. Finch OLIN SEWALL PETTINGILL, JR.: AN APPRECIATION...........................................................14 Paul Baicich MAMMALS IN GREAT HORNED OWL PELLETS FROM BOULDER COUNTY, COLORADO............16 Rebecca E. Marvil and Alexander Cruz UPCOMING CFO FIELD TRIPS.........................................................................................23 THE SHRIKES OF DEARING ROAD, EL PASO COUNTY, COLORADO 1993-2001....................24 Susan H. Craig RING-BILLED GULLS FEEDING ON RUSSIAN-OLIVE FRUIT...................................................32 Nicholas Komar NEWS FROM THE C OLORADO BIRD R ECORDS COMMITTEE (JANUARY 2002).........................35 Tony Leukering NEWS FROM THE FIELD: THE SUMMER 2001 REPORT (JUNE - JULY)...................................36 Christopher L. Wood and Lawrence S. Semo COLORADO F IELD O -

The Costs of Reproduction in Tree Swallows (Tachycineta Bicolor)L
2540 The costs of reproduction in tree swallows (Tachycineta bicolor)l NATHANIEL T. WHEELWRIGHT; JOANNA LEARY, AND CARAGH FITZGERALD Department of Biology, Bowdoin CIJ/lege,Brunswick. ME 04011, U.S.A. Received June 20, 1990 WHEELWRIGHT,N. T., LEARY, J., and FITZGERALD,C. 1991. The costs of reproduction in tree swallows (Tachycineta bicolor). Can. J. Zool. 69: 2540-2547. We investigated the effect of brood size on nestling growth and survival, parental survival, and future fecundity in tree swallows (Tach)cineta bicolor) over a 4-year period (1987 -1990) in an effort to understand whether reproductive trade-offs limit clutch size in birds. In addition to examining naturally varying brood sizes in a population on Kent Island, New Brunswick, Canada, we experimentally modified brood sizes, increasing or decreasing the reproductive burdens of females by two offspring. Unlike previous studies, broods of the same females were enlarged or reduced in up to 3 successive years in a search for evidence of cumulative costs of reproduction that might go undetected by a single brood manipulation. Neither observation nor experiment supported the existence of a trade-off between offspring quality and quantity, in contrast with the predictions of life-history theory. Nestling wing length, mass, and tarsus length were unrelated to brood size. Although differences between means were in the direction predicted, few differences were statistically significant, despite large sample sizes.~estlings from small broods were no more likely to return as breeding adults than nestlings from large broods, but return rates of both groups were very low. Parental return rates were also independent of brood size, and there was no evidence of a negati ve effect of brood size on future fecundity (laying date, clutch size). -

Biolphilately Vol-62 No-4
100 Biophilately June 2014 Vol. 63 (2) ORNITHOLOGY Editor Glenn G. Mertz, BU1455 New Issues As I prepared to write this article for April, May, and June 2014, /LQQ¶V6WDPS1HZV laid a bomb on their readers ZLWKWKHQRWLILFDWLRQWKDWWKH\KDYHHOLPLQDWHGWKH³1HZ,VVXHV´VHFWLRQLQWKHLUZHHNO\SXEOLFDWLRQ What this means is that collectors and dealers will not be alerted as to what issues are in the pipeline from the some 180+ countries of the globe by their postal authorities. This also means that, by no longer listing this information that was part of my listing, details such as designer, printing method, or printing entity will no longer appear as part of the stamp listing thDW,SURYLGHIRUHDFKLVVXH,VWLOOKDYHVRPHRIWKHVHDUWLFOHVIURPSULRU/LQQ¶VOLVWLQJVIRU issues I have not yet reported, but eventually I will no longer be able to provide this information. Another concern is not knowing when a bird stamp is part of a mixed stamp issue, because Scott Publishing Company does not always list a description of every stamp in a mixed stamp issue as to the subject matter. I have UHDGDQGVHHQDQXPEHURIOHWWHUVWR/LQQ¶VE\VXEVFULEHUVUHJDUGLQJWKHHOLPLQDWLRQRIWKH³1HZ,VVXHV´VHFWLRQ /LQQ¶VVWDWHGUHDVRQIRUHOLPLQDWLRQRQSDJHRIWKH)HEUXDU\ZHHNO\LVVXHLVWKHWLPHLWWRRNWRJHWWKHVH listings and that it furthermore was a duplication of the content in the once-a-PRQWK³6FRWW1HZ,VVXH8SGDWH´ listing in the /LQQ¶V6WDPS1HZV. It appears that /LQQ¶V6WDPS1HZV is not aware of how far the new issue updates are behind in listing some countries. A few are six or more years behind and other are about one year. The losers in this decision are the stamp collecting community and maybe the dealers. And they wonder why stamp collecting is waning as a hobby. -

TREE SWALLOW Tachycineta Bicolor Tree Swallows Are the Only Swallows to Be Seen All Year in the Sacramento Region
TREE SWALLOW Tachycineta bicolor Tree swallows are the only swallows to be seen all year in the Sacramento region. A few winter here; others come to join them early in the spring. They are about sparrow-length but slimmer. The male is steel blue on top, white underneath. Tree swallows like wooded areas near streams. They can be seen skimming close to the water scooping up insects. Usually a hole in a tree trunk is chosen for a nest and lined with grass, leaves and feathers. The violet green swallow, a bird with similar colors but with white around the sides of the rump, is found here in warm weather. CLIFF SWALLOW Petrochelidon pyrrhonota Cliff swallows, famous for their annual return to San Juan Capistrano, likewise return to the Sacramento region within a few days of the same date each year. Wherever a bridge or culvert houses a colony of these swallows, the same kind of homecoming takes place each spring. Cliff swallows are identified by their buff foreheads, orange rumps, and square tails. They fly with long sweeping glides and steep climbs, catching flying insects on the wing. The bulb-shaped nests, hung under bridges, highway overpasses and eaves of buildings, are built entirely of mud. Flocks of cliff swallows can be seen around puddles gathering mud in their bills. When disturbed by an intruder or when flying over feeding grounds, the birds utter a squeaky, guttural sound. BARN SWALLOW Hirundo rustica Here is a bird with a real “swallow tail” outfit. In fact, it is our only swallow with a deeply forked tail. -

Tree Swallow Taehyeineta Hie%R
Tree Swallow Taehyeineta hie%r Tree Swallows are among the most familiar Vermont birds. Their breeding habitat is usually, but not always, located near water; it includes farmland, wet meadows, marshes, and brooks, as well as villages near mead ows, streams, or ponds. Nests are often placed in dead snags that project above the water of a lake or pond. The Tree Swallow's affinity for water habitats is related to the abundant insect life in such areas. The other major requirement for nesting habitat is the presence of cavities suitable for nest sites primarily natural cavities, old woodpecker predators by circling over and diving at in holes, and nesting boxes. Although highly truders while uttering a sharp, clicking call territorial at the nest site, Tree Swallows (Stokes 1979). The nest, built by the female, sometimes nest in close proximity if suitable is a collection of dry grasses or pine needles nest boxes or trees with old woodpecker lined with feathers, 90% of which are white. holes are present. Single pairs will some Tree Swallows are sometimes observed fly times take up residence at remote beaver ing low over a domestic duck and plucking ponds. a feather from its back (M. Metcalf, pers. Swallows are active birds and are found observ.). Several pairs nesting near each in open habitats where observation is not other may compete for feathers. Nest build difficult. The Tree Swallow, widely distrib ing may take as long as a month, but average uted in Vermont, was found in 178 of the construction time is 2 to 3 weeks (Stokes 179 Atlas Project priority blocks. -

A Guide to the Bird Notes of Grace Mccormac French of Yamhill County, Oregon
Studies In OregonOrnithology N o.2 A Guide to the BirdNotes of Grace M cCo r mac French of Yamhill County, Oregon Range D.Bayer SWALLOWS ARE BACK Mrs. French on Albino Birds To the Editor: Whilewe were In commentinglately uponthe suburban eatingSunday breakfasta tele- phone message came announc- advent of analbinorobin it was suggested on ing the arrival of the swallows this pagethatsuchbirdsare more or less in the yard of Mr. and Mrs. feathered rela- Howard Coleman on the shore made outcastby their nornielly of Carlton lake. This year two tives. This particular specimen,for, instance, tree swallows were the firstar- never was seen with.companions. But a letter rivals. Some yearsthe earliest from thebird woman, Grace MacCormac dateisfortheviolet-green swallow.My spring records for French,who usedto keep tabs on the, swal- thefirstswallowsbeganin lows near Carlton, but now lives at` Dayton; 1915. It might interest you to know tells us that she has known albino robins that I have another albino to add to my list. It was a junco GardenClub Hears with white body and pearl gray head and tail. It fed in the yard BirdLore Talk between December22 and Jan- uary17. By Mrs. French GRACE McC. FRENCH, Mrs. Grace MacCormac French, P. O. Box471, Dayton. recognizedasanauthorityon birds, spoke beforethe MZMinn- ville Garden Club at their meet- ingMonday, 'March17.In addi- 8tgns of the Seasons tiontospeakingon theday's topic, "How To AttractBirds", Mrs. French told of many of her By Grace McCormac French observationsand amusinginci- The springmigrationofthe dents which occurredduring the birds always attracts much atten- thirty-two yearsthey resided on tion. -
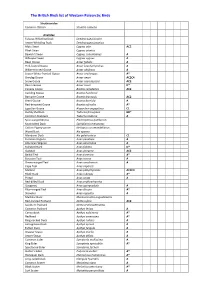
BB List 2016V1
The British Birds list of Western Palearctic Birds Struthionidae Common Ostrich Struthio camelus Anatidae Fulvous Whistling Duck Dendrocygna bicolor Lesser Whistling Duck Dendrocygna javanica Mute Swan Cygnus olor AC2 Black Swan Cygnus atratus Bewick’s Swan Cygnus columbianus A Whooper Swan Cygnus cygnus A Bean Goose Anser fabalis A Pink-footed Goose Anser brachyrhynchus A White-fronted Goose Anser albifrons A Lesser White-fronted Goose Anser erythropus A* Greylag Goose Anser anser AC2C4 Snow Goose Anser caerulescens AC2 Ross’s Goose Anser rossii D* Canada Goose Branta canadensis AC2 Cackling Goose Branta hutchinsii Barnacle Goose Branta leucopsis AC2 Brent Goose Branta bernicla A Red-breasted Goose Branta ruficollis A* Egyptian Goose Alopochen aegyptiaca C1 Ruddy Shelduck Tadorna ferruginea BD* Common Shelduck Tadorna tadorna A Spur-winged Goose Plectropterus gambensis Knob-billed Duck Sarkidiornis melanotos Cotton Pygmy-goose Nettapus coromandelianus Wood Duck Aix sponsa Mandarin Duck Aix galericulata C1 Eurasian Wigeon Anas penelope A American Wigeon Anas americana A Falcated Duck Anas falcata D* Gadwall Anas strepera AC2 Baikal Teal Anas formosa A* Eurasian Teal Anas crecca A Green-winged Teal Anas carolinensis A Cape Teal Anas capensis Mallard Anas platyrhynchos AC2C4 Black Duck Anas rubripes A* Pintail Anas acuta A Red-billed Duck Anas erythrorhyncha Garganey Anas querquedula A Blue-winged Teal Anas discors A* Shoveler Anas clypeata A Marbled Duck Marmaronetta angustirostris Red-crested Pochard Netta rufina AC2 Southern Pochard