Unitypoint Health Comment on COVID-19 Pandemic Response
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2021-2022 Custom & Standard Information Due Dates
2021-2022 CUSTOM & STANDARD INFORMATION DUE DATES Desired Cover All Desired Cover All Delivery Date Info. Due Text Due Delivery Date Info. Due Text Due May 31 No Deliveries No Deliveries July 19 April 12 May 10 June 1 February 23 March 23 July 20 April 13 May 11 June 2 February 24 March 24 July 21 April 14 May 12 June 3 February 25 March 25 July 22 April 15 May 13 June 4 February 26 March 26 July 23 April 16 May 14 June 7 March 1 March 29 July 26 April 19 May 17 June 8 March 2 March 30 July 27 April 20 May 18 June 9 March 3 March 31 July 28 April 21 May 19 June 10 March 4 April 1 July 29 April 22 May 20 June 11 March 5 April 2 July 30 April 23 May 21 June 14 March 8 April 5 August 2 April 26 May 24 June 15 March 9 April 6 August 3 April 27 May 25 June 16 March 10 April 7 August 4 April 28 May 26 June 17 March 11 April 8 August 5 April 29 May 27 June 18 March 12 April 9 August 6 April 30 May 28 June 21 March 15 April 12 August 9 May 3 May 28 June 22 March 16 April 13 August 10 May 4 June 1 June 23 March 17 April 14 August 11 May 5 June 2 June 24 March 18 April 15 August 12 May 6 June 3 June 25 March 19 April 16 August 13 May 7 June 4 June 28 March 22 April 19 August 16 May 10 June 7 June 29 March 23 April 20 August 17 May 11 June 8 June 30 March 24 April 21 August 18 May 12 June 9 July 1 March 25 April 22 August 19 May 13 June 10 July 2 March 26 April 23 August 20 May 14 June 11 July 5 March 29 April 26 August 23 May 17 June 14 July 6 March 30 April 27 August 24 May 18 June 15 July 7 March 31 April 28 August 25 May 19 June 16 July 8 April 1 April 29 August 26 May 20 June 17 July 9 April 2 April 30 August 27 May 21 June 18 July 12 April 5 May 3 August 30 May 24 June 21 July 13 April 6 May 4 August 31 May 25 June 22 July 14 April 7 May 5 September 1 May 26 June 23 July 15 April 8 May 6 September 2 May 27 June 24 July 16 April 9 May 7 September 3 May 28 June 25. -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -

Flex Dates.Xlsx
1st Day 1st Day of Your Desired Stay you may Call January 3, 2021 ↔ November 4, 2020 January 4, 2021 ↔ November 5, 2020 January 5, 2021 ↔ November 6, 2020 January 6, 2021 ↔ November 7, 2020 January 7, 2021 ↔ November 8, 2020 January 8, 2021 ↔ November 9, 2020 January 9, 2021 ↔ November 10, 2020 January 10, 2021 ↔ November 11, 2020 January 11, 2021 ↔ November 12, 2020 January 12, 2021 ↔ November 13, 2020 January 13, 2021 ↔ November 14, 2020 January 14, 2021 ↔ November 15, 2020 January 15, 2021 ↔ November 16, 2020 January 16, 2021 ↔ November 17, 2020 January 17, 2021 ↔ November 18, 2020 January 18, 2021 ↔ November 19, 2020 January 19, 2021 ↔ November 20, 2020 January 20, 2021 ↔ November 21, 2020 January 21, 2021 ↔ November 22, 2020 January 22, 2021 ↔ November 23, 2020 January 23, 2021 ↔ November 24, 2020 January 24, 2021 ↔ November 25, 2020 January 25, 2021 ↔ November 26, 2020 January 26, 2021 ↔ November 27, 2020 January 27, 2021 ↔ November 28, 2020 January 28, 2021 ↔ November 29, 2020 January 29, 2021 ↔ November 30, 2020 January 30, 2021 ↔ December 1, 2020 January 31, 2021 ↔ December 2, 2020 February 1, 2021 ↔ December 3, 2020 February 2, 2021 ↔ December 4, 2020 1st Day 1st Day of Your Desired Stay you may Call February 3, 2021 ↔ December 5, 2020 February 4, 2021 ↔ December 6, 2020 February 5, 2021 ↔ December 7, 2020 February 6, 2021 ↔ December 8, 2020 February 7, 2021 ↔ December 9, 2020 February 8, 2021 ↔ December 10, 2020 February 9, 2021 ↔ December 11, 2020 February 10, 2021 ↔ December 12, 2020 February 11, 2021 ↔ December 13, 2020 -

COVID-19 Dashboard
6/4/2020 Public Health Indicators Massachusetts Department of Public Health COVID-19 Dashboard - Thursday, June 04, 2020 Dashboard of Public Health Indicators Newly Reported Total Cases Cases Today Below is the status as of June 1, 2020: 471 102,063 Indicator Measure Status Newly Reported Total Deaths Deaths Today 1 COVID-19 positive test rate ⚫ 2 Number of individuals who died from COVID-19 ⚫ 50 7,201 3 Number of patients with COVID-19 in hospitals ⚫ New Patients Total Patients 4 Healthcare system readiness ⚫ Tested by Tested by 5 Testing capacity ⚫ Molecular Tests Molecular Tests 6 Contact tracing capabilities ⚫ 7,115 621,248 Legend New Patients Total Patients Tested by Tested by Antibody Tests Antibody Tests 1,022 48,436 1 1/1 6/4/2020 Confirmed v Probable Massachusetts Department of Public Health COVID-19 Dashboard - Thursday, June 04, 2020 Confirmed and Probable Case Breakdown Confirmed Probable Newly Reported Total Confirmed Newly Reported Total Probable Confirmed Cases Cases Probable Cases Cases Today Today 412 98,376 59 3,687 Newly Reported Total Deaths Newly Reported Total Deaths Deaths among among Confirmed Deaths among among Probable Confirmed Today Cases Probable Today Cases 50 7,062 0 139 Patients with a positive molecular test for COVID-19 are counted as confirmed. Patients with a positive serology/antibody test and either COVID-like symptoms or likely exposure to COVID-19 are counted as probable cases. Patients who did not have a laboratory test but whose death certificate listed COVID-19 as a cause of death are counted as probable deaths. Probable cases are included in all counts from March 1 onward. -

BLUE Contract Teacher Calendar CALENDAR LEGEND DATES
2020-21 REGULAR SCHOOL CALENDAR School Board approved on 11/05/2019 Revised on 2/10/2021 BLUE Contract Teacher July 2020 DA TES/EVENTS M T W Th F 196 197 201 Aug 10 Education University Option #1 – Calendar 1 2 3 for Year 5-8 BLUE Teachers only 6 7 8 9 10 13 14 15 16 17 (196 day contract) CALENDAR LEGEND 20 21 22 23 24 Aug 10 First Day to Report – Year 1 BLUE 27 28 29 30 31 Teachers (201 day contract) and August 2020 Year 2-4 BLUE Teachers (197 day Professional Development M T W Th F 3 4 5 6 7 contract) 10 11 12 13 14 1 2 4 Aug 11 First Day to Report – Year 5-8 BLUE Education University Staff 17 18 19 20 21 3 4 8 Teachers (196 day contract) 24 25 26 27 28 8 9 13 Development Aug 12-13** Year 1 BLUE Teachers do not 31 9 10 14 September 2020 report Holidays-Paid M T W Th F Aug 12-18 Year 2-8 BLUE Teachers do not 1 2 3 4 13 14 18 (No classes; offices closed) report 7 8 9 10 11 18 19 23 Aug 19 Education University Option #2 14 15 16 17 18 23 24 28 Conference Comp Day (No 21 22 23 24 25 28 29 33 Aug 20-Sept 4 Professional Development Days classes; paid break) 28 29 30 31 32 36 October 2020 Sept 7 Labor Day Holiday First/Last Day of School M T W Th F 1 2 33 34 38 Sept 8 First day of school for students 5 6 7 8 9 38 39 43 First/Last Day for Staff (see 12 13 14 15 16 43 44 48 Oct 30 ½ Prof Dev, ½ Parent Teacher details in Dates/Events) 19 20 21 22 23 48 49 53 26 27 28 29 30 53 54 58 Conference November 2020 Oct 30 –Nov 3 Fall Break for Students Break-Paid (No classes) M T W Th F 2 3 4 5 6 56 57 61 Nov 2-3 Fall Break for Staff Break-Unpaid (No -

2018 - 2019 Days of Rotation Calendar
2018 - 2019 DAYS OF ROTATION CALENDAR Day # Date Rotation Day Type Notes Day # Date Rotation Day Type Notes Saturday, October 13, 2018 Sunday, October 14, 2018 Monday, September 3, 2018 Holiday/Vaca Labor Day 27 Monday, October 15, 2018 Day 3 In Session 1 Tuesday, September 4, 2018 Day 1 In Session 28 Tuesday, October 16, 2018 Day 4 In Session 2 Wednesday, September 5, 2018 Day 2 In Session 29 Wednesday, October 17, 2018 Day 5 In Session 3 Thursday, September 6, 2018 Day 3 In Session 30 Thursday, October 18, 2018 Day 6 In Session 4 Friday, September 7, 2018 Day 4 In Session 31 Friday, October 19, 2018 Day 1 In Session Saturday, September 8, 2018 Saturday, October 20, 2018 Sunday, September 9, 2018 Sunday, October 21, 2018 Monday, September 10, 2018 Day Holiday/Vaca Rosh Hashanah 32 Monday, October 22, 2018 Day 2 In Session 5 Tuesday, September 11, 2018 Day 5 In Session 33 Tuesday, October 23, 2018 Day 3 In Session 6 Wednesday, September 12, 2018 Day 6 In Session 34 Wednesday, October 24, 2018 Day 4 In Session 7 Thursday, September 13, 2018 Day 1 In Session 35 Thursday, October 25, 2018 Day 5 In Session 8 Friday, September 14, 2018 Day 2 In Session 36 Friday, October 26, 2018 Day 6 In Session Saturday, September 15, 2018 Saturday, October 27, 2018 Sunday, September 16, 2018 Sunday, October 28, 2018 9 Monday, September 17, 2018 Day 3 In Session 37 Monday, October 29, 2018 Day 1 In Session 10 Tuesday, September 18, 2018 Day 4 In Session 38 Tuesday, October 30, 2018 Day 2 In Session Wednesday, September 19, 2018 Day Holiday/Vaca Yom Kippur 39 Wednesday, October 31, 2018 Day 3 In Session 11 Thursday, September 20, 2018 Day 5 In Session 40 Thursday, November 1, 2018 Day 4 In Session 12 Friday, September 21, 2018 Day 6 In Session 41 Friday, November 2, 2018 Day 5 In Session Saturday, September 22, 2018 Saturday, November 3, 2018 Sunday, September 23, 2018 Sunday, November 4, 2018 13 Monday, September 24, 2018 Day 1 In Session 42 Monday, November 5, 2018 Day 6 In Session 14 Tuesday, September 25, 2018 Day 2 In Session Tuesday, November 6, 2018 Prof Dev. -

Julian Date Cheat Sheet for Regular Years
Date Code Cheat Sheet For Regular Years Day of Year Calendar Date 1 January 1 2 January 2 3 January 3 4 January 4 5 January 5 6 January 6 7 January 7 8 January 8 9 January 9 10 January 10 11 January 11 12 January 12 13 January 13 14 January 14 15 January 15 16 January 16 17 January 17 18 January 18 19 January 19 20 January 20 21 January 21 22 January 22 23 January 23 24 January 24 25 January 25 26 January 26 27 January 27 28 January 28 29 January 29 30 January 30 31 January 31 32 February 1 33 February 2 34 February 3 35 February 4 36 February 5 37 February 6 38 February 7 39 February 8 40 February 9 41 February 10 42 February 11 43 February 12 44 February 13 45 February 14 46 February 15 47 February 16 48 February 17 49 February 18 50 February 19 51 February 20 52 February 21 53 February 22 54 February 23 55 February 24 56 February 25 57 February 26 58 February 27 59 February 28 60 March 1 61 March 2 62 March 3 63 March 4 64 March 5 65 March 6 66 March 7 67 March 8 68 March 9 69 March 10 70 March 11 71 March 12 72 March 13 73 March 14 74 March 15 75 March 16 76 March 17 77 March 18 78 March 19 79 March 20 80 March 21 81 March 22 82 March 23 83 March 24 84 March 25 85 March 26 86 March 27 87 March 28 88 March 29 89 March 30 90 March 31 91 April 1 92 April 2 93 April 3 94 April 4 95 April 5 96 April 6 97 April 7 98 April 8 99 April 9 100 April 10 101 April 11 102 April 12 103 April 13 104 April 14 105 April 15 106 April 16 107 April 17 108 April 18 109 April 19 110 April 20 111 April 21 112 April 22 113 April 23 114 April 24 115 April -

2021 Subdivision Calendar
Planning Department Development Review – Major Subdivision Applications First Plat Review 2021 Deadlines Submittal to Staff Sufficiency TRC Meeting Date Deadline for Staff Planning Board BOC Meeting Planning Department Review Deadline Postponement Meeting Date* Date** December 18, 2020 December 31 January 13 January 22 February 2 March 15 January 15 January 29 February 10 February 19 March 2 April 19 February 19 March 5 March 17 March 26 April 6 May 17 March 19 April 2 April 14 April 23 May 4 June 21 April 16 April 30 May 12 May 21 June 1 July 19 May 21 June 4 June 16 June 25 July 6 August 16 June 18 July 2 July 14 July 23 August 3 September 20 July 23 August 6 August 18 August 27 September 7 October 18 August 20 September 3 September 15 September 24 October 5 November 15 September 17 October 1 October 13 October 22 November 2 December 20 October 22 November 5 November 17 November 26 December 7 ▪ January 18, 2022 November 19 December 3 December 15 ▪ December 22 ▪ January 4, ▪ February 21, 2022 2022 ▪ ▪ ▪ December 17 December 31, January 12, ▪ January 21, ▪ February 1, March 21, 2021 2022 2022 2022 2022 * Planning Board Has Two (2) Meetings to Make Recommendation ** Board of Commissioners Has Up to Four (4) Meetings to Make Decision ▪ 2021 Dates are Tentative – Meeting Dates Will Be Set in December 2021 With Adoption of 2022 Meeting Calendars Construction/Final Plat Review – Administrative* 2021 Deadlines Submittal to Staff Sufficiency TRC Meeting Date Planning Review Deadline Department For TRC December 23, 2020 January 6 January 13 January -

Pay Date Calendar
Pay Date Information Select the pay period start date that coincides with your first day of employment. Pay Period Pay Period Begins (Sunday) Pay Period Ends (Saturday) Official Pay Date (Thursday)* 1 January 10, 2016 January 23, 2016 February 4, 2016 2 January 24, 2016 February 6, 2016 February 18, 2016 3 February 7, 2016 February 20, 2016 March 3, 2016 4 February 21, 2016 March 5, 2016 March 17, 2016 5 March 6, 2016 March 19, 2016 March 31, 2016 6 March 20, 2016 April 2, 2016 April 14, 2016 7 April 3, 2016 April 16, 2016 April 28, 2016 8 April 17, 2016 April 30, 2016 May 12, 2016 9 May 1, 2016 May 14, 2016 May 26, 2016 10 May 15, 2016 May 28, 2016 June 9, 2016 11 May 29, 2016 June 11, 2016 June 23, 2016 12 June 12, 2016 June 25, 2016 July 7, 2016 13 June 26, 2016 July 9, 2016 July 21, 2016 14 July 10, 2016 July 23, 2016 August 4, 2016 15 July 24, 2016 August 6, 2016 August 18, 2016 16 August 7, 2016 August 20, 2016 September 1, 2016 17 August 21, 2016 September 3, 2016 September 15, 2016 18 September 4, 2016 September 17, 2016 September 29, 2016 19 September 18, 2016 October 1, 2016 October 13, 2016 20 October 2, 2016 October 15, 2016 October 27, 2016 21 October 16, 2016 October 29, 2016 November 10, 2016 22 October 30, 2016 November 12, 2016 November 24, 2016 23 November 13, 2016 November 26, 2016 December 8, 2016 24 November 27, 2016 December 10, 2016 December 22, 2016 25 December 11, 2016 December 24, 2016 January 5, 2017 26 December 25, 2016 January 7, 2017 January 19, 2017 1 January 8, 2017 January 21, 2017 February 2, 2017 2 January -

195 Revised 12/20
2020 — 2021 NORMAN PUBLIC SCHOOLS 2020 — 2021 LENGTH OF CONTRACT Beginning Date: July 24, 2020 NUMBER OF NON-DUTY DAYS NUMBER OF VACATION DAYS HOLIDAYS X 195 Ending Date: June 3, 2021 66 0 0 STAFF - NON-DUTY DAYS OPAT Parent Educator July 1-3, 6-10, 13-17, 20-23 Nov 23-27 Mar 15-19 Fine Arts Secretary (Sec #2) Aug Dec 21-25, 28-31 Apr Site Athletic/Activities Director Sept 7 Jan 1, 4, 18 May 31 Oct 8-9 Feb June 4, 7-11, 14-18, 21-25, 28-30 July 2020 August 2020 September 2020 Sun Mon Tue Wed Thur Fri Sat Sun Mon Tue Wed Thur Fri Sat Sun Mon Tue Wed Thur Fri Sat 1 2 3X 4 1 1 2 3 4 5 5 6 7 8 9 10 11 2 3 4 5 6 7 8 6 7X 8 9 10 11 12 SICK LEAVE 12 13 14 15 16 17 18 9 10� 11� 12� 13� 14� 15 13 14 15 16 17 18 19 19 20 21 22 23 24 25 16 17� 18� 19� 20� 21� 22 20 21 22 23 24 25 26 10 26 27 28 29 30 31 23 24❤ 25 26 27 28 29 27 28 29 30 � 30 31 NUMBER OF DAYS October 2020 November 2020 December 2020 WORKED PER MONTH Sun Mon Tue Wed Thur Fri Sat Sun Mon Tue Wed Thur Fri Sat Sun Mon Tue Wed Thur Fri Sat 1 2 3 1 2 3 4 5 6 7 1 2 3 4 5 July 6 4 5 6 7 8 9 10 8 9 10 11 12 13 14 6 7 8 9 10 11 12 Aug 21 11 12 13 14 15 16 17 15 16 17 18 19 20 21 13 14 15 16 17 18❤ 19 Sept 21 18 19 20 21 22 (23) 24 22 23 24 25 26X 27 28 20 21 22 23 24 25X 26 ❤ Oct 16 27SD 28SD 29SD 30SD 25 26❤ 31 29 30 27 28 29 30 31 Nov 16 Dec 14 January 2021 February 2021 March 2021 Jan 18 Sun Mon Tue Wed Thur Fri Sat Sun Mon Tue Wed Thur Fri Sat Sun Mon Tue Wed Thur Fri Sat Feb 20 1X 2 1 2 3 4 5 6 1 2 3 4 5 6 Mar 18 3 /4/ [5] 6❤ 7 8 9 7 8 9 10 11 12 13 7 8 9 10 11 (12)❤ 13 Apr 22 10 11 -
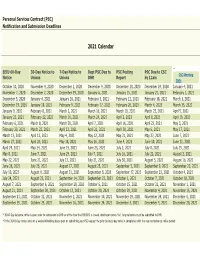
2021 Calendar
Personal Services Contract (PSC) Notification and Submission Deadlines 2021 Calendar SEIU 60-Day 30-Day Notice to 7-Day Notice to Dept PSC Due to PSC Posting PSC Due to CSC CSC Meeting Notice Unions Unions DHR Report by 11am Date October 10, 2020 November 9, 2020 December 1, 2020 December 9, 2020 December 18, 2020 December 24, 2020 January 4, 2021 November 7, 2020 December 7, 2020 December 29, 2020 January 6, 2021 January 15, 2021 January 21, 2021 February 1, 2021 December 5, 2020 January 4, 2021 January 26, 2021 February 3, 2021 February 12, 2021 February 18, 2021 March 1, 2021 December 19, 2020 January 18, 2021 February 9, 2021 February 17, 2021 February 26, 2021 March 4, 2021 March 15, 2021 January 9, 2021 February 8, 2021 March 2, 2021 March 10, 2021 March 19, 2021 March 25, 2021 April 5, 2021 January 23, 2021 February 22, 2021 March 16, 2021 March 24, 2021 April 2, 2021 April 8, 2021 April 19, 2021 February 6, 2021 March 8, 2021 March 30, 2021 April 7, 2021 April 16, 2021 April 22, 2021 May 3, 2021 February 20, 2021 March 22, 2021 April 13, 2021 April 21, 2021 April 30, 2021 May 6, 2021 May 17, 2021 March 13, 2021 April 12, 2021 May 4, 2021 May 12, 2021 May 21, 2021 May 27, 2021 June 7, 2021 March 27, 2021 April 26, 2021 May 18, 2021 May 26, 2021 June 4, 2021 June 10, 2021 June 21, 2021 April 24, 2021 May 24, 2021 June 15, 2021 June 23, 2021 July 2, 2021 July 8, 2021 July 19, 2021 May 8, 2021 June 7, 2021 June 29, 2021 July 7, 2021 July 16, 2021 July 22, 2021 August 2, 2021 May 22, 2021 June 21, 2021 July 13, 2021 July 21, 2021 July -

April-June 2021
APRIL-JUNE 2021 DATES MONDAY TUESDAY WEDNESDAY THURSDAY FRIDAY NO CLASSES: March 29 - Periods 5 - 7 Periods 1 - 4 ACT Testing (Juniors only) Periods 5 - 7 Periods 1 - 4 Mandamur Service April 2 Student Activities (TBD) NO CLASSES: April 5 - 9 Professional Periods 1 - 4 Periods 5 - 7 Periods 1 - 4 Periods 5 - 7 Development Day NO CLASSES: April 12 - 16 Periods 1 - 4 SAT Testing (Juniors only) Periods 5 - 7 Periods 1 - 4 Periods 5 - 7 Student Activities (TBD) April 19 - 23 Monday Schedule 1-7 Periods 1 - 4 Periods 5 - 7 Periods 1 - 4 Periods 5 - 7 April 26 - 30 Monday Schedule 1-7 Periods 1 - 4 Periods 5 - 7 Periods 1 - 4 Periods 5 - 7 Periods 5 - 7 May 3 - 7 Monday Schedule 1-7 Periods 1 - 4 Periods 5 - 7 Periods 1 - 4 Crowning of Mary May 10 - 14 Monday Schedule 1-7 Periods 1 - 4 Periods 5 - 7 Periods 1 - 4 Periods 5 - 7 NO CLASSES: May 17 - 21 Professional Periods 1 - 4 Periods 5 - 7 Periods 1 - 4 Periods 5 - 7 Development Day May 24 - 28 Monday Schedule 1-7 Periods 1 - 4 Periods 5 - 7 Periods 1 - 4 Periods 5 - 7 May 31 - June 4 NO CLASSES Seniors’ last week Periods 1 - 4 Periods 5 - 7 Periods 1 - 4 Periods 5 - 7 Memorial Day Graduation: June 6 June 7 - 11 Periods 5 - 7 NO CLASSES: Periods 1 - 4 Periods 5 - 7 Periods 1 - 4 9-11 grades only END OF SEMESTER 2 Teacher Work Day June 14 - 18 SCHOOL OUT! *There is no “final exam” week this year.