Interim Report on Testing and Tracing Special Committee on Covid-19 Response August 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
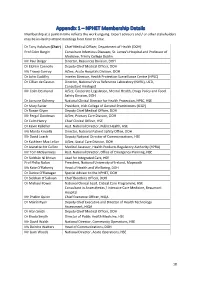
Appendix 1 – NPHET Membership Details Membership at a Point in Time Reflects the Work Ongoing
Appendix 1 – NPHET Membership Details Membership at a point in time reflects the work ongoing. Expert advisors and / or other stakeholders may be invited to attend meetings from time to time. Dr Tony Holohan (Chair) Chief Medical Officer, Department of Health (DOH) Prof Colm Bergin Consultant Infectious Diseases, St. James’s Hospital and Professor of Medicine, Trinity College Dublin Mr Paul Bolger Director, Resources Division, DOH Dr Eibhlin Connolly Deputy Chief Medical Officer, DOH Ms Tracey Conroy A/Sec, Acute Hospitals Division, DOH Dr John Cuddihy Interim Director, Health Protection Surveillance Centre (HPSC) Dr Cillian de Gascun Director, National Virus Reference Laboratory (NVRL), UCD, Consultant Virologist Mr Colm Desmond A/Sec, Corporate Legislation, Mental Health, Drugs Policy and Food Safety Division, DOH Dr Lorraine Doherty National Clinical Director for Health Protection, HPSC, HSE Dr Mary Favier President, Irish College of General Practitioners (ICGP) Dr Ronan Glynn Deputy Chief Medical Officer, DOH Mr Fergal Goodman A/Sec, Primary Care Division, DOH Dr Colm Henry Chief Clinical Officer, HSE Dr Kevin Kelleher Asst. National Director, Public Health, HSE Ms Marita Kinsella Director, National Patient Safety Office, DOH Mr David Leach Deputy National Director of Communications, HSE Dr Kathleen Mac Lellan A/Sec, Social Care Division, DOH Dr Jeanette Mc Callion Medical Assessor, Health Products Regulatory Authority (HPRA) Mr Tom McGuinness Asst. National Director, Office of Emergency Planning, HSE Dr Siobhán Ní Bhrian Lead for -

1 National Public Health Emergency Team – COVID-19 Meeting Note
National Public Health Emergency Team – COVID-19 Meeting Note – Standing meeting Date and Time Thursday 7th January 2021, (Meeting 71) at 10:00am Location Department of Health, Miesian Plaza, Dublin 2 Chair Dr Tony Holohan, Chief Medical Officer, DOH Dr Ronan Glynn, Deputy Chief Medical Officer, DOH Dr Kevin Kelleher, Assistant National Director, Public Health, HSE Prof Philip Nolan, President, National University of Ireland, Maynooth and Chair of the Irish Epidemiological Modelling Advisory Group (IEMAG) Dr Cillian de Gascun, Laboratory Director, NVRL and Expert Advisory Group (EAG) Chair Dr Máirín Ryan, Deputy Chief Executive and Director of HTA, HIQA Dr John Cuddihy, Interim Director, HSE HPSC Dr Siobhán Ní Bhriain, Lead for Integrated Care, HSE Dr Eibhlín Connolly, Deputy Chief Medical Officer, DOH Dr Mary Favier, Immediate past president of the ICGP, Covid-19 advisor Dr Michael Power, Consultant in Anaesthetics / Intensive Care Medicine, Beaumont Hospital Ms Rachel Kenna, Chief Nursing Officer, DOH Mr Greg Dempsey, Deputy Secretary, Governance and Performance Division, DOH Ms Tracey Conroy, Assistant Secretary, Acute Hospitals Policy Division, DOH Dr Lorraine Doherty, National Clinical Director Health Protection, HSE Members via Dr Colette Bonner, Deputy Chief Medical Officer, DOH videoconference1 Mr Colm Desmond, Assistant Secretary, Corporate Legislation, Mental Health, Drugs Policy and Food Safety Division, DOH Ms Yvonne O’Neill, National Director, Community Operations, HSE Mr Phelim Quinn, Chief Executive Officer, HIQA Dr Siobhán -

Irish Universities Help F Ight the Covid-19 Pandemic
IRISH UNIVERSITIES HELP FIGHT THE COVID-19 PANDEMIC www.iua.ie @IUAofficial Irish Universities help fight the COVID-19 Pandemic Doctors Samer Arnous, Tony Moloney and Nick Barrett at University Hospital Limerick, testing University of Limerick produced visors and shield box. Exec Summary The COVID-19 pandemic has created unprecedented societal challenges. The Irish university sector has maintained ‘business as usual’ to the greatest extent possible by a rapid transition to remote learning and assessment. Meanwhile, the sector galvanised into immediate action, contributing to the national emergency response in every way possible as the pandemic developed. Our universities and their staff and students have, and are, making a hugely valuable contribution to the national efforts to fight the COVID-19 pandemic. We have captured key highlights in this publication. A more comprehensive schedule can be found at https://www.iua.ie/covid-19/universities-help- fight-the-pandemic/ Irish Universities help fight the COVID-19 Pandemic 3 Here are the key highlights of university efforts: Page 07 1 Page 11 2 COVID-19 testing Expert advice with staff and contact tracing: and students on the frontline: Highly skilled diagnostic laboratory staff from our Expert advice has been the hallmark of dealing with universities have been readily mobilised to undertake the COVID-19 crisis. Leading academics from across laboratory processing of samples and to take swabs the university sector have been on hand to guide and from patients at testing hubs. support the response: • Our researchers rose to the challenge of the • University leaders such asMaynooth University scarcity of testing reagents with our labs rallying to President, Philip Nolan and University College produce lysis buffer, viral transport medium and Dublin’s Dr Cillian de Gascun, have headed up key other essential solutions. -

Dáil Éireann
DÁIL ÉIREANN AN COMHCHOISTE UM SHLÁINTE JOINT COMMITTEE ON HEALTH Dé Céadaoin, 23 Meitheamh 2021 Wednesday, 23 June 2021 Tháinig an Comhchoiste le chéile ag 9.30 a.m. The Joint Committee met at 9.30 a.m. Comhaltaí a bhí i láthair/Members present: Teachtaí Dála/Deputies Seanadóirí/Senators Colm Burke, Martin Conway, Cathal Crowe, Annie Hoey, David Cullinane, Seán Kyne. Bernard J. Durkan, Gino Kenny, John Lahart. Teachta/Deputy Seán Crowe sa Chathaoir/in the Chair. 1 JH Business of Joint Committee Chairman: Apologies have been received from Deputy Hourigan and Senator Black. Be- fore we commence our formal proceedings, we need to agree the draft minutes for last week’s meeting of 16 June, which have been circulated. Are they agreed? Agreed. Update on the Cyberattack, Covid-19 Vaccination Roll-out and Covid-19 Restrictions in Maternity Hospitals: Health Service Executive Chairman: I welcome the witnesses to our meeting this morning. They will provide an update on the recent cyberattack, the Covid-19 vaccination roll-out and the public health re- strictions in maternity hospitals. From the HSE, we have Mr. Paul Reid, chief executive officer; Ms Anne O’Connor, chief operations officer, Dr. Colm Henry, chief clinical officer; Mr. Fran Thompson, chief information officer; and Mr. Damien McCallion, national lead of the Covid-19 vaccination programme. Before we hear their opening statements, I need to point out to our witnesses that there is uncertainty as to whether parliamentary privilege will apply to evidence that is given from a location outside the parliamentary precincts of Leinster House. -

High Level Task Force on COVID-19 Vaccination 21St December 2020
High Level Task Force on COVID-19 Vaccination 21st December 2020 Meeting Updates, decisions and actions from meeting High Level Taskforce on COVID-19 Vaccination | 21st December 2020 Meeting High Level Task Force on COVID-19 Vaccination Monday 21st December 2020 14:00 Updates, decisions and actions arising from meeting 1. Attendees A. Members in attendance B. Additional attendees in support Prof. Brian MacCraith, Task Force Chair i. Task Force Secretariat Dr Tony Holohan, Chief Medical Officer, DOH Kate Waterhouse, Task Force Secretariat Fergal Goodman, Assistant Secretary, Health ii. In Attendance Protection Division, DOH Liz Canavan, Chair, Senior Officials Group on Dr Lorraine Doherty, Clinical Director Health COVID-19 Protection, HSE Paul Reid, Chief Executive Officer, HSE Sean Bresnan, National Director of Procurement, HSE Dr Colm Henry, Chief Clinical Officer, HSE Gerry O’Brien, Director, Health Protection, DOH Paul Quinn, Government CPO and CEO, OGP Deirdre Watters, Head of Communications, DOH Barry Lowry, Chief Information Officer, OGCIO Lucy Jessop, SRO Director, National Immunisation Office, HSE Dermot Mulligan, Assistant Secretary, Innovation David Walsh, SRO WS4 - Vaccine Process & and Investment Division, DETE Workforce Derek McCormack, Expert on Cold Chain Logistics John Cuddihy, SRO WS5 - Surveillance, Monitoring and Reporting Dalton Philips, Chief Executive Officer, DAA Fran Thompson, SRO WS6 - Enabling Technology & Information Lorraine Nolan, Chief Executive, Health Products Michael Lohan, IDA Regulatory Authority (HPRA) Rachel Kenna, Chief Nursing Officer, DOH iii. Programme support Prof Karina Butler, Chair, National Immunisation Michael McDaid (PWC), Programme Office Advisory Committee (NIAC) Derek Tierney, Programme Director Yvonne Mowlds (PWC), Programme Office 2 High Level Taskforce on COVID-19 Vaccination | 21st December 2020 Meeting 2. -

Dr Tony Holohan Chief Medical Officer Chair of the National Public Health Emergency Team Department of Health Miesian Plaza Dublin 2 Vía Email: [email protected] C.C
Morrison Chambers 32 Nassau Street Dublin 2 D02 YE06 01 679 3188 Dr Tony Holohan Chief Medical Officer Chair of the National Public Health Emergency Team Department of Health Miesian Plaza Dublin 2 Vía email: [email protected] c.c. Professor Martin Cormican; HSE, Dr Ronan Glynn, Department of Health Vía email: [email protected], [email protected] Wednesday, 26 March 2021 Dear Dr Holohan, Firstly, let me extend my condolences to you on the death of your wife last month. I hope you are doing well and managing during these extraordinary times. We write concerning the restrictions imposed at this time on funerals and the rituals that surround dying in Ireland. Bereavement is intrinsically lonely, COVID-19 restrictions have made it more isolating. It has reduced people’s general social outlets, working patterns and natural support networks. The current restrictions of allowing only 10 people attend any aspect of a funeral is causing additional stress, anxiety and heartache to many families. We see the current restrictions as being overly harsh and should be revisited as we learn to live with COVID-19 and manage infection risks. A recent survey, carried out on our behalf, indicates that over 75% of people feel that the grief impact of the pandemic on the nation will be long-lasting. For bereaved individuals, planning funerals of loved ones, honouring that person and receiving the support of their extended family and friends makes for a good impact – we would be keen to minimise any negative impacts over time. Funerals by their nature are made up of both indoor and outdoor elements. -

1 National Public Health Emergency Team – COVID-19
National Public Health Emergency Team – COVID-19 Meeting Note – Standing meeting Date and Time Thursday 25th February 2021, (Meeting 78) at 10:00am Location Department of Health, Miesian Plaza, Dublin 2 Chair Dr Ronan Glynn, Deputy Chief Medical Officer, DOH Dr Kevin Kelleher, Assistant National Director, Public Health, HSE Prof Philip Nolan, President, National University of Ireland, Maynooth and Chair of the Irish Epidemiological Modelling Advisory Group (IEMAG) Dr Cillian de Gascun, Laboratory Director, NVRL and Expert Advisory Group (EAG) Chair Dr Máirín Ryan, Deputy Chief Executive and Director of HTA, HIQA Dr John Cuddihy, Interim Director, HSE HPSC Prof Colm Bergin, Consultant in Infectious Diseases, St James’s Hospital Dr Siobhán Ní Bhriain, Lead for Integrated Care, HSE Dr Eibhlín Connolly, Deputy Chief Medical Officer, DOH Dr Mary Favier, Immediate past president of the ICGP, Covid-19 advisor Dr Michael Power, Consultant in Anaesthetics / Intensive Care Medicine, Beaumont Hospital Ms Rachel Kenna, Chief Nursing Officer, DOH Ms Tracey Conroy, Assistant Secretary, Acute Hospitals Policy Division, DOH Dr Lorraine Doherty, National Clinical Director Health Protection, HSE Dr Colette Bonner, Deputy Chief Medical Officer, DOH Ms Yvonne O’Neill, National Director, Community Operations, HSE Members via Mr Phelim Quinn, Chief Executive Officer, HIQA videoconference1 Dr Darina O’Flanagan, Special Advisor to the NPHET Mr Fergal Goodman, Assistant Secretary, Primary Care Division, DOH Dr Breda Smyth, Public Health Specialist, HSE Dr Kathleen -

Mr. Paul Reid, Chief Executive Officer & Chair HSE National Crisis
Mr. Paul Reid, Chief Executive Officer & Chair HSE National Crisis Management Team (NCMT), Health Services Executive, Dr Steevens’ Hospital, Dublin 8, D08 W2A8 18th January 2021 Via email to: [email protected] Dear Paul, Following the recent meeting of the COVID-19 National Public Health Emergency Team (NPHET), I wish to bring to your attention, as Chair of the HSE NCMT, the following decision of the NPHET which is now required to be actioned by the HSE and Health Protection Surveillance Centre (HPSC): • The NPHET endorsed the proposals for testing of close contacts as set out within “Review of duration of restriction of movements for individuals exposed, or potentially exposed, to Covid-19 (via close contact with a confirmed case only)”, to include: o The recommencement of testing of close contacts amongst the general public at day 5, as soon as swabbing, testing and contact tracing capacity can facilitate this development; o Close contacts of any positive case who has travelled from Great Britain, South Africa or Brazil should continue to be requested to self-isolate and get a test at day 5; o The urgent implementation of day 5 and day 10 testing for HCWs designated as close contacts, with exit from restricted movements if the Day 10 test is reported as ‘not detected’ I would like to take this opportunity to thank you and the wider team across the HSE for your ongoing support and work across the health and social care services as we move through the COVID-19 National Public Health Emergency. Officials from this Department have been and continue to be available to work with relevant HSE staff. -

1 National Public Health Emergency Team – COVID-19 Meeting Note
National Public Health Emergency Team – COVID-19 Meeting Note Date and Time Sunday 4th October 2020, (Meeting 57) at 12:00pm Location Department of Health, Miesian Plaza, Dublin 2 Chair Dr Tony Holohan, Chief Medical Officer, DOH Dr Ronan Glynn, Deputy Chief Medical Officer, DOH Dr Kevin Kelleher, Assistant National Director, Public Health, HSE Prof Philip Nolan, President, National University of Ireland, Maynooth and Chair of the Irish Epidemiological Modelling Advisory Group (IEMAG) Dr Cillian de Gascun, Laboratory Director, NVRL and Expert Advisory Group (EAG) Chair Dr Máirín Ryan, Deputy Chief Executive and Director of HTA, HIQA Dr John Cuddihy, Interim Director, HSE HPSC Prof Colm Bergin, Consultant in Infectious Diseases, St James’s Hospital Dr Michael Power, Consultant in Anaesthetics / Intensive Care Medicine, Beaumont Hospital Dr Eibhlín Connolly, Deputy Chief Medical Officer, DOH Dr Mary Favier, Immediate past president of the ICGP, Covid-19 advisor Ms Tracey Conroy, Assistant Secretary, Acute Hospitals Policy Division, DOH Dr Siobhán O’Sullivan, Chief Bioethics Officer, DOH Dr Colette Bonner, Deputy Chief Medical Officer, DOH Mr Colm Desmond, Assistant Secretary, Corporate Legislation, Mental Health, Drugs Policy and Food Safety Division, DOH Members via Mr Phelim Quinn, Chief Executive Officer, HIQA videoconference Dr Darina O’Flanagan, Special Advisor to the NPHET Mr Fergal Goodman, Assistant Secretary, Primary Care Division, DOH Dr Breda Smyth, Public Health Specialist, HSE Mr Tom McGuinness, Assistant National Director -

Face-Masks and the Limits of “Evidence-Based” Health Policy Dr
Face-masks and the limits of “evidence-based” health policy Dr. Louise Caffrey argues that there is good enough evidence to support making face coverings compulsory, if you look beyond what’s strictly medical During the Covid-19 pandemic the Health Information and Quality Authority (HIQA) has been advising the Clinical Expert Advisory Group in supporting the National Public Health Emergency Team. Most recently, HIQA reviewed the evidence on the use of face covering to prevent people from transmitting the Covid-19 virus. Current policy is to only recommend the public wear face coverings rather than making them compulsory on, for example, public transport, in shops and in services. Particularly as we move out of lockdown, whether or not to mandate face coverings is a key question since we now know that the virus is spread, not just through coughing or sneezing, but also by speaking. People are contagious for days before they display symptoms and some people who are contagious never display symptoms. For this reason there is a need to stop people who have no idea they are sick giving the virus to others, for example, when they go to the supermarket or use public transport or indeed if they work in a shop or prepare food for the public. The government’s decision not to make face coverings in enclosed public spaces compulsory is highly controversial because face coverings are most effective at stopping people transmitting the virus. Face coverings are much less effective at stopping people who wear them from getting the virus. So for face coverings to be most effective, and for those wearing them to be well protected, they need to be worn by most people. -

APRIL – JUNE 2020 Date Received Our Ref. 2020 Summary of Request
APRIL – JUNE 2020 Date Our Summary of Request Category Decision Decision Received Ref. of Date Made 2020 Requester 30/06/2020 222 Records which document spend on mental health care via private services outside Business 30/09/2020 Part Granted the HSE i) within Ireland & ii) outside Ireland from 1 Jan 2018 to 31 May 2020. Interest Group 24/06/2020 221 Correspondence between the Department and DPER/DEASP on any review of Journalist 21/07/2020 Refused enhanced illness benefit in relation to covid-19 and possible consequences for the private healthcare worker workforce 24/06/2020 220 Letters and emails (including attachments) between CMO Tony Holohan and HSE Journalist 22/07/2020 Granted Chief Clinical Officer Colm Henry from April 1 to April 25, 2020. 24/06/2020 219 Representations to the Minister for Health, and the Minister's replies, in relation to Journalist 21/07/2020 Part Granted the Children & Family Relationships Act (2015), during the period Feb 1, 2014 to April 6, 2015 and the period Feb 1, 2019 to Feb 29, 2020 24/06/2020 218 Submissions made to the Department of Health in relation to the Assisted Human Journalist 31/07/2020 Part Granted Reproduction legislation during the period January 1, 2017 to January 31, 2020, 24/06/2020 217 Costs of production and distribution of both the Irish & English language Covid-19 Journalist 10/07/2020 Part Granted public information booklet + correspondences re; the initial Covid-19 public information booklet not being bilingual. 24/06/2020 216 Breakdown of details and costs on radio/television advertisements, social media Business 10/07/2020 Refused posts and adverts produced in English and Irish re; Covid-19 information. -

Dáil Éireann
Vol. 762 Tuesday, No. 3 24 April 2012 DÍOSPÓIREACHTAÍ PARLAIMINTE PARLIAMENTARY DEBATES DÁIL ÉIREANN TUAIRISC OIFIGIÚIL—Neamhcheartaithe (OFFICIAL REPORT—Unrevised) Dé Máirt, 24 Aibreán 2012. Ceisteanna — Questions Minister for Public Expenditure and Reform Priority Questions …………………………… 277 Other Questions …………………………… 286 Leaders’ Questions ……………………………… 296 Topical Issue Matters ……………………………… 304 Ceisteanna — Questions (resumed) Other Questions (resumed) The Taoiseach …………………………… 305 Estimates for Public Services 2012: Messages from Select Committees …………… 319 Order of Business ……………………………… 319 Ministerial Rota for Parliamentary Questions: Motion ………………… 326 Topical Issue Debate Pension Provisions …………………………… 327 Nursing Home Repayment Scheme ……………………… 329 Departmental Reports …………………………… 331 Garda Stations ……………………………… 333 Social Welfare and Pensions Bill 2012: Second Stage (resumed)……………………………336 Motion to Instruct Committee ………………………… 344 Private Members’ Business Motorist Emergency Relief Bill 2012: Second Stage ………………… 357 Questions: Written Answers …………………………… 379 DÁIL ÉIREANN ———— Dé Máirt, 24 Aibreán 2012. Tuesday, 24 April 2012. ———— Chuaigh an Ceann Comhairle i gceannas ar 2.00 p.m. ———— Paidir. Prayer. ———— Ceisteanna — Questions Priority Questions ———— Sale of State Assets 107. Deputy Sean Fleming asked the Minister for Public Expenditure and Reform if he will ensure that the sale of State assets will not include the disposal of any assets of a company involved in the provision of water supply to households throughout the country; and if he will make a statement on the matter. [20542/12] Minister for Public Expenditure and Reform (Deputy Brendan Howlin): As the House will be aware, the Government decided last week that the new Irish water utility is to be an indepen- dent State-owned subsidiary of Bord Gáis Éireann. The new water utility is not and will not be included in the Government’s programme of State asset disposals.