Malignant Proliferating Pilar Tumors Arising in KID Syndrome: a Report of Two Patients
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dermatologists' Perceptions on the Utility and Limitations
RESEARCH/Original Article Journal of Telemedicine and Telecare 2021, Vol. 27(3) 166–173 Dermatologists’ perceptions on the ! The Author(s) 2019 utility and limitations of teledermatology Article reuse guidelines: sagepub.com/journals-permissions after examining 55,000 lesions DOI: 10.1177/1357633X19864829 journals.sagepub.com/home/jtt Mara Giavina Bianchi , Andre Santos and Eduardo Cordioli Abstract Introduction: Few studies have assessed the perception of teledermatologists about the utility and limitations of teledermatology, especially to diagnose a broad range of skin diseases. This study aimed to evaluate dermatologists’ confidence in teledermatology, its utility and limitations for dermatological conditions in primary care. Methods: An analytical study that used a survey for dermatologists who diagnosed 30,916 patients with 55,012 lesions through teledermatology during a 1-year project in S~ao Paulo, Brazil. Results: Dermatologists found teledermatology useful for triage and diagnosis, especially for xerotic eczema, pigmen- tary disorders and superficial infections. Their confidence in teledermatology was statistically higher by the end of the project (p ¼ 0.0012). Limitations included some technical issues and the impossibility to suggest how soon the patient should be assisted face-to-face by a dermatologist. The most treatable group of diseases by teledermatology was superficial infections (92%). The use of dermoscopy images would significantly increase the confidence to treat atypical naevi and malignant tumours (p < 0.0001 and p ¼ 0.0003 respectively). Follow-ups by teledermatology or feedback from primary-care physicians would be desirable, according to the dermatologists. Discussion: We found it interesting that dermatologists became increasingly confident in teledermatology after the project and how they classified teledermatology as useful for triage, diagnosis and even treatment of most types of skin conditions followed at primary care. -

Proliferating Trichilemmal Cyst of the Scalp: Nine Cases and Literature Review
Otorhinolaryngology-Head and Neck Surgery Research Article ISSN: 2398-4937 Proliferating trichilemmal cyst of the scalp: Nine cases and literature review ElBenaye J1,3*, Sinaa M2,3, Elkhachine Y1,3, Sakkah A1,3, Jakar A1 and Elhaouri M1 1Department of Dermatology, Moulay Ismail Military Hospital, Meknes, Morocco 2Department of Cytopathology, Moulay Ismail Military Hospital, Meknes, Morocco 3Department of Medicine and Pharmacy, Sidi Mohammed Ben Abdellah University (USMBA), Fes, Morocco Abstract Background: proliferating trichilemmal cyst (PTC) is a rare adnexal tumor, primarily sitting on the scalp of elderly women. Its evolution is generally benign despite the rare malignant cases described. We report a series of 9 cases of which one is malignant and metastatic. Material and methods: We retrospectively reviewed data of all patients with PTC seen at department of dermatology in the Meknes military hospital, between January 2013 and December 2017. Results: Nine cases of PTC were diagnosed, which 8 in elderly women, with 62 years mean age. Trauma was found in one third of cases correlated with rapid growth of the tumor. The latter was symptomatic in 2/3 of the cases. Ulceration involved only the malignant case. The size varied from 1,5 to 12 cm. Recurrence after surgery was noted in 2 cases (malignant tumor and multi-cystic tumor). Discussion: PTC appears to have a well-distinguished profile of the simple trichilemmal cyst. Some clinical and histological features may constitute prognostic factors of aggressiveness. Histological and immunohistochemical study is crucial in the management. A large excision remains the only guarantee of complete remission without recurrence or metastasis. -

2016 Essentials of Dermatopathology Slide Library Handout Book
2016 Essentials of Dermatopathology Slide Library Handout Book April 8-10, 2016 JW Marriott Houston Downtown Houston, TX USA CASE #01 -- SLIDE #01 Diagnosis: Nodular fasciitis Case Summary: 12 year old male with a rapidly growing temple mass. Present for 4 weeks. Nodular fasciitis is a self-limited pseudosarcomatous proliferation that may cause clinical alarm due to its rapid growth. It is most common in young adults but occurs across a wide age range. This lesion is typically 3-5 cm and composed of bland fibroblasts and myofibroblasts without significant cytologic atypia arranged in a loose storiform pattern with areas of extravasated red blood cells. Mitoses may be numerous, but atypical mitotic figures are absent. Nodular fasciitis is a benign process, and recurrence is very rare (1%). Recent work has shown that the MYH9-USP6 gene fusion is present in approximately 90% of cases, and molecular techniques to show USP6 gene rearrangement may be a helpful ancillary tool in difficult cases or on small biopsy samples. Weiss SW, Goldblum JR. Enzinger and Weiss’s Soft Tissue Tumors, 5th edition. Mosby Elsevier. 2008. Erickson-Johnson MR, Chou MM, Evers BR, Roth CW, Seys AR, Jin L, Ye Y, Lau AW, Wang X, Oliveira AM. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011 Oct;91(10):1427-33. Amary MF, Ye H, Berisha F, Tirabosco R, Presneau N, Flanagan AM. Detection of USP6 gene rearrangement in nodular fasciitis: an important diagnostic tool. Virchows Arch. 2013 Jul;463(1):97-8. CONTRIBUTED BY KAREN FRITCHIE, MD 1 CASE #02 -- SLIDE #02 Diagnosis: Cellular fibrous histiocytoma Case Summary: 12 year old female with wrist mass. -

Histopathological Profile of Surgically Excised Scalp and Skull Lesions Asuman Kilitci¹ , Ziya Asan²
DOI: 10.5152/cjms.2018.442 Original Article Histopathological Profile of Surgically Excised Scalp and Skull Lesions Asuman Kilitci¹ , Ziya Asan² 1Department of Pathology, Ahi Evran University School of Medicine, Kırşehir, Turkey ²Department of Neurosurgery, Ahi Evran University School of Medicine, Kırşehir, Turkey ORCID ID of the author: A.K. 0000-0002-5489-2222; Z.A. 0000-0001-8468-9156 Cite this article as: Kilitci A, Asan Z. Histopathological Profile of Surgically Excised Scalp and Skull Lesions. Cyprus J Med Sci 2018; 3: 63-7. BACKGROUND/AIMS Although subcutaneous lesions of the scalp are more common than those of the skull, few studies in literature have assessed the frequency of scalp and skull diseases. The goal of this study was to establish the frequency and main histopathological findings of these lesions. Our study is one of the largest series and shows that the incidence of surgically excised scalp masses includes an array of diseases. MATERIAL and METHODS We reviewed 265 extracranial masses from 173 patients. The mean age of the patients, gender distribution, localization and characteristics of lesions, histopathological type and radiological features were analyzed. RESULTS One-hundred (57.8%) patients were males and 73 (42.2%) were females. The mean age was 42.98 (range, 5-87). In total, 261 were within the scalp, 1 involved the scalp and skull and 3 were within the limits of the skull. Six lesions exhibited malignant features. There were 101 trichilemmal cysts; 74 epidermal cysts; 38 intradermal nevus; 8 verruca vulgaris; -

Histopathology Squamous Cell Carcinoma
Squamous cell carcinoma - Mohs Ron Rapini MD No conflict of interest Josey Chair, Dept Dermatology • I am paid zero to speak at this Univ Texas Medical School at Houston MD Anderson Cancer Center course, other than travel • The tuition helps to run the ASMS programs Duplicate Actinic keratosis Actinic keratosis Rx • Whether you call it a “precancer” or • Treating orAKs is beyond this lecture “squamous cell carcinoma grade 1/2” or • Ill-defined dysplasias – usually a “field-effect” “SCC – AK type”, or “keratinocytic • Trying to eradicate totally is like playing intraepithelial neoplasia = KIN”, it still “whack a mole” so goal is to eradicate most doesn’t need Mohs surgery significant areas • Don’t over-read as invasive squamous cell ca • Cryo, curettage, laser, imiquimod, • AK and squamous cell carcinoma are often fluorouracil, diclofenac, etc multifocal with field effects in margins Keratinocytic intraepithelialDistribute Grading dysplasia in neoplasia = KIN 1, 2, 3 dysplastic nevi is analogous • Analogous to CIN, PIN, VIN 1,2,3 • Really in SKIN with most popular • NIH consensus conference 1992 system, we don’t have a “2”: recommended grading cytology despite Not lack of concordance AK = KIN-1 • I grade only cytology as mild, moderate, SCC in situ (Bowen’s) = KIN-3 Do severe 1 Lack of concordance on grading “dysplasia” in dysplastic nevi Other terms Piepkorn (J Cutan Pathol 6:542, 1992) • Bowenoid AK – “just call it found only 38% agreement SCCis?” Similar situation with KIN-2 (in between • Advanced AK – “beware” AK and SCC in situ) – -

Cysts a Cyst Is a Benign, Round, Dome-Shaped Encapsulated Lesion
Cysts A cyst is a benign, round, dome-shaped encapsulated lesion that contains fluid or semi-fluid material. It may be firm or fluctuant and often distends the overlying skin. There are several types of cyst. The most common are described here. Pseudocyst? Cysts that are not surrounded by a capsule are better known as pseudocysts. These commonly arise in acne. Cysts Epidermoid cysts are due to the proliferation of epidermal cells within the dermis. So basically the layers of skin that are supposed to be on the outside instead invaginate in. Skin is tucked in to form a sack that is lined by healthy epidermal cells that continue to multiply, mature and form keratin. These can be found just about anywhere on the body. A central pore or punctum may be present. Keratinous contents are soft, cheese-like and malodorous. The origin of a trichilemmal cyst is hair root sheath and, thus, these are often found on the scalp and are very firm. Inheritance is autosomal dominant. The origin of steatocystoma is the sebaceous duct within the hair follicle. A solitary steatocystoma is known as steatocystoma simplex. More often, there are multiple lesions (steatocystoma multiplex) on the chest, upper arms, axillae, neck and scrotum or vulva and this is usually an autosomal dominantly inherited disorder. The cysts arise in the late teens and 20s due to the effect of androgens and persist lifelong. They are freely moveable, smooth flesh to yellow color papules 3–30 mm in diameter. There is no central punctum. The content of the cysts is predominantly sebum. -

Thomas, Md Associate Professor Section of Dermatopathology Valencia Thomas, Md Ut Houston School of Medicine Associate Professor Mohs and Dermasurgery Unit M.D
11/7/2016 HISTOPATHOLOGY OF SQUAMOUS CELL CARCINOMA VALENCIA THOMAS, MD ASSOCIATE PROFESSOR SECTION OF DERMATOPATHOLOGY VALENCIA THOMAS, MD UT HOUSTON SCHOOL OF MEDICINE ASSOCIATE PROFESSOR MOHS AND DERMASURGERY UNIT M.D. ANDERSON CANCER CENTER DERMATOPATHOLOGY, UNIVERSITY OF TEXAS HEALTH SCIENCE CENTER AT HOUSTON DIRECTOR, MOHS SURGERY, M.D. ANDERSON CANCER CENTER Duplicate or Distribute Not Actinic Squamous Invasive Keratosis cell squamous Do carcinoma in- cell situ carcinoma 1 11/7/2016 ACTINIC KERATOSIS ATYPICAL KERATINOCYTIC PROLIFERATIONS • Actinic keratosis (AK) • “precancer” • Partial-thickness keratinocytic atypia • “squamous cell carcinoma grade 1/2” • Squamous cell carcinoma in-situ • “SCC – AK type” • Full-thickness keratinocytic atypia • Invasive squamous cell carcinoma • “keratinocytic intraepithelial neoplasia = KIN” • Well-differentiated • Does not need Mohs surgery • Moderately-differentiated • Poorly-differentiated • AK and squamous cell carcinoma are often • Other modifiers multifocal with field effects in margins • Depth of invasion, perineural invasion, lymphovascular • 1/20 develop into a skin cancer invasion, Duplicate KERATINOCYTIC INTRAEPITHELIAL AK: PARTIAL-THICKNESS ATYPIA NEOPLASIA = KIN 1, 2, 3 or • Analogous to CIN, PIN, VIN 1,2,3 • Really in SKIN with most popular system, we don’t have a “2”: AK = KIN-1 SCC in situ (Bowen’s) = KIN-3 Distribute ACTINIC KERATOSIS RX AK: PARTIAL-THICKNESS ATYPIA • Treating AKs is beyond this lecture • Trying to eradicateNot totally is like playing “whack a mole” so goal is -

Global Development Assistance for Adolescent Health from 2003 to 2015
Supplementary Online Content Li Z, Li M, Patton GC, Lu C. Global development assistance for adolescent health from 2003 to 2015. JAMA Netw Open. 2018;1(4):e181072. doi:10.1001/jamanetworkopen.2018.1072 eTable 1. List of Donor Countries Included in the CRS eTable 2. 132 Recipients in the CRS (According to the World Health Organization Regions) eTable 3. Key Words to Identify the Related Age Group (Adolescence) in the Creditor Reporting System eTable 4. CRS Purpose Name and Respective Fractions Allocated to Adolescent Health eTable 5. Definitions of DAAH on the Leading Causes of DALYs of Adolescent Health eTable 6. Key Words Used to Search for Projects on Skin and Subcutaneous Diseases in the Creditor Reporting System eTable 7. Key Words Used to Search for Road Injury Projects in the Creditor Reporting System eTable 8. Key Words Used to Search for HIV/AIDS Projects in the Creditor Reporting System eTable 9. Key Words Used to Search for Projects on Iron-Deficiency Anemia in the Creditor Reporting System eTable 10. Key Words Used to Search for Self-Harm Projects in the Creditor Reporting System eTable 11. Key Words Used to Search for Projects on Interpersonal Violence in the Creditor Reporting System eTable 12. Key Words Used to Search for Projects on Depressive Disorders in the Creditor Reporting System eTable 13. Key Words Used to Search for Projects on Lower Back and Neck Pain in the Creditor Reporting System eTable 14. Key Words Used to Search for Diarrheal Projects in the Creditor Reporting System eTable 15. Key Words Used to Search for Tuberculosis Projects in the Creditor Reporting System eTable 16. -

Squamous Cell Carcinoma Complicated a Trichilemmal Cyst
erm d D ato an lo y g g ic o l D El Jouari et al., J Dermatol Dis 2018, 5:2 o t i s a e Dermatology and Dermatologic DOI: 10.4172/2376-0427.1000277 a m s r e e s D Diseases: Open Access ISSN: 2376-0427 Letter to Editor Open Access Squamous Cell Carcinoma Complicated A Trichilemmal Cyst Ouiame El Jouari*, Sara Elloudi, Rime Dassouly, Ghita Senhaji, Zakia Douhi, Hanane Baybay and Fatima Zahra Mernissi Department of Dermatology, University Hospital Hassan II, Fez, Morocco *Corresponding author: Ouiame El Jouari, Department of Dermatology, University Hospital Hassan II, Fez, Morocco, Tel: 212677879830; E-mail: [email protected] Received date: November 28, 2018; Accepted date: December 20, 2018; Published date: December 28, 2018 Copyright: ©2018 El Jouari O, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Letter to the Editor Dear editor, Trichilemmal cyst, also known as pilar cyst, is a dermal epithelial cyst developed at the expense of the hair follicle. It affects Caucasian women with a mean age of 60 years more frequently [1]. They are most often asymptomatic, localized mainly on the scalp. These tumors are often benign but local recurrences are sometimes aggressive. The risk of malignant change of trichilemmal cyst is rare. We describe an uncommon case of squamous cell carcinoma complicated a trichilemmal cyst. A 70-years old patient with 20 years’ history of painless nodular Figure 2: Dermoscopy showing yellowish structures, linear vessels scalp lesions, progressively increasing in number and size. -
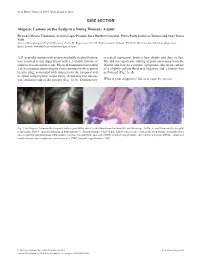
QUIZ SECTION Alopecic Lesions on the Scalp in A
Acta Derm Venereol 2015 Epub ahead of print QUIZ SECTION Alopecic Lesions on the Scalp in a Young Woman: A Quiz Elena del Alcazar Viladomiu, Arantxa López Pestaña, Sara Ibarbia Oruezabal, Maria Paula Gutiérrez Tamara and Anna Tuneu Valls Servicio Dermatología, Hospital Donostia, Paseo Dr. Begiristain 107-116, Edificio Amara 2ªplanta, ES-20014 Donostia-San Sebastián, (Gipuzkoa), Spain. E-mail: [email protected] A 21-year-old woman with an unremarkable medical history revealed numerous broken hair shafts and fine vellus. was referred to our department with a 2-month history of She did not report any itching or pain associated with the alopecic lesions on her scalp. Physical examination revealed lesions and had no systemic symptoms. Bacterial culture 2 skin-coloured, dome-shaped cystic tumours with a central of a slightly yellow fluid was negative and a biopsy was keratin plug, associated with alopecia on the temporal and performed (Fig. 1c, d). occipital scalp regions, respectively. A melanocytic naevus was seen near one of the patches (Fig. 1a, b). Dermoscopy What is your diagnosis? See next page for answer. Fig. 1. (a) Alopecic lesion on the temporal scalp region with a raised central tumour and melanocytic naevus on top. (b) The second lesion on the occipital scalp region. Note a central keratin plug in both tumours. (c) Histopathology: a dilated hair follicle with a cystic cavity in the deep dermis, surrounded by a non-necrotizing granulomatous inflammatory reaction (haematoxylin and eosin (H&E), original magnification ×40). (d) Deep dermal infiltrate, comprised mostly of numerous lymphocytes and histiocytes (H&E, original magnification × 200). -

Proliferating Trichilemmal Cyst with Lymphadenopathy: a Discussion of Cutaneous Neoplasms Involving the Scalp
Central Journal of Ear, Nose and Throat Disorders Bringing Excellence in Open Access Case Study *Corresponding author James Paul Dworkin-Valenti, Department of Otolaryngology, Head & Neck Surgery, Detroit Medical Proliferating Trichilemmal Center, 5807 Cedar Ridge Dr, Ann Arbor, MI 48103, USA, Tel: 313-966-9406; 248-444-7396; Email: Cyst with Lymphadenopathy: Submitted: 22 April 2016 Accepted: 29 April 2016 A Discussion of Cutaneous Published: 02 May 2016 Copyright Neoplasms Involving the Scalp © 2016 Valenti et al. OPEN ACCESS Richard T. Klapchar1, Billie Jean Crigger2 and James Paul Dworkin-Valenti3* 1Department of Otolaryngology, Head & Neck Surgery, Doctors Hospital, USA 2Private Practice, Owensboro, USA 3Department of Otolaryngology, Head & Neck Surgery, Detroit Medical Center, USA Abstract This paper introduces a unique case example of a trichilemmal scalp cyst that was initially erroneously diagnosed as a cancerous lesion because of its proliferating cellular morphology. The evaluation and treatment histories of this patient are augmented by a detailed review of the literature on this condition, especially with respect to cases of rare malignant transformation to squamous cell carcinoma. Whereas proliferating pilar or trichilemmal cysts are uncommon tumors that rarely undergo malignant transformation [1]. Squamous cell carcinomas with metastasis to regional head and neck lymph nodes rarely develop from proliferating pilar cysts. However, such degeneration has been reported in the literature [2]. These lesions are fraught with significant morbidity and mortality, and their treatment usually consists of substantial surgical excision. Histopathologic evidence of malignancy often demands adjuvant regimens of radiation therapy (+/-chemotherapy) [3]. The purpose of this case report is to present a patient with an ulcerative, necrotic scalp mass and lymphadenopathy whose initial biopsy demonstrated squamous cell carcinoma. -

Giant Solitary Trichilemmal Cyst of the Upper Eye Lid: Masquerading Lacrimal Gland Tumor: a Clinico Radio Pathological Case Report
Case Report JOJ Ophthal Volume 4 Issue 2 - August 2017 Copyright © All rights are reserved by Anita Panda DOI: 10.19080/JOJO.2017.04.555633 Giant Solitary Trichilemmal Cyst of the Upper Eye Lid: Masquerading Lacrimal Gland Tumor: A Clinico Radio Pathological Case Report Anita P1*, Nandita C1 and Saita2 1Department of Ophthalmology, Sharda Hospital, India 2Department of Pathology, India Submission: July 21, 2017; Published: August 21, 2017 *Corresponding author: Anita Panda, Department of Ophthalmology, Sharda Hospital, Greater Noida, India, Email: Abstract Purpose: To report a case of giant solitary trichilemmal cyst in a 58-year-old man. Design: Observational case report. Methods: A 58-year-old man sought treatment for a mass in the right upper eyelid. The lesion was excised and examined histopathologically. Results: without granular cell layer. There was no atypia, mitosis, or stromal invasion. Light microscopy showed a cystic lesion lined by stratified squamous epithelium with a compact layer of eosinophilic keratin Conclusion: trichilemmal cyst masquerading as Lacrimal Gland Tumor which was managed by surgical excission. Solitary trichilemmal cyst is rare in comparison to multiple cyst. To the best of our knowledge, this is the first case report of Introduction of the mass. There is drooping of upper eye lid since then. He is Trichilemmal cyst, also known as a pilar cyst, forms from a non vegeterian and none of his family members had similar the outer root sheath of hair follicle. They are common benign problem. An ophthalmic examination revealed a visual acuity tumors most often found on the scalp [1]. The involvement of of 20/40 in each eye due to presence of cataract which was not improved with pin hole or refraction [9-10].