Synthesis of 1-Hydroxyethyl-2-Dimethyl Alkylammonium Surfactants and Their Properties in Aqueous Solution
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

United States Patent (19) 11 Patent Number: 5,883,058 Wells Et Al
USOO5883058A United States Patent (19) 11 Patent Number: 5,883,058 Wells et al. (45) Date of Patent: *Mar 16, 1999 54 HIGH LATHER STYLING SHAMPOOS 4,784,801 11/1988 Hoeffkes et al. ....................... 252/554 5,084.212 1/1992 Farris et al. ............................ 252/554 (75) Inventors: Robert Lee Wells, Cincinnati, Ohio; 5,104,642 4/1992 Wells et al. ..... ... 424/47 Jon Robert Behrens, Kobe, Japan 5,120,532 6/1992 Wells et al. ............ ... 424/70 5,310,508 5/1994 Subramanyam et al. ............... 252/549 73) Assignee: The Procter & Gamble Company, 5,391,368 2/1995 Gerstein ............................... 424/70.13 5,514,302 5/1996 Brown ..................................... 252/545 Cincinnati, Ohio 5,580,494 12/1996 Sandhu et al. .......................... 510/125 Notice: The term of this patent shall not extend FOREIGN PATENT DOCUMENTS beyond the expiration date of Pat. No. 5,672.576. 0323715 12/1989 European Pat. Off.. Appl. No.: 520,631 Primary Examiner Paul Lieberman Assistant Examiner Necholas Ogden Filed: Aug. 29, 1995 Attorney, Agent, or Firm Joan B. Tucker; William J. Int. Cl." ................................................ C110 1/83 Winter; Tara M. Rosnell U.S. Cl. .......................... 510/127; 510/119,510/123; 57 ABSTRACT 510/125; 424/70.11; 424/70.24 The present invention relates to hair shampoo compositions Field of Search ..................................... 252/549, 550, which have improved cleansing, lathering, and Styling ben 252/551, 557; 510/119, 123,125, 127; efits=. These Shampoo compositions comprise an alkyl glyc 424/70.24, 70.11 eryl ether Sulfonate Surfactant, a hair Styling polymer, a 56) References Cited non-polar volatile Solvent, and water. -

A Study of Interaction of Cetyltrimethyl Ammonium Bromide with Methyl Red by Conductometry in Methanol-Water Mixed Solvent Media
A STUDY OF INTERACTION OF CETYLTRIMETHYL AMMONIUM BROMIDE WITH METHYL RED BY CONDUCTOMETRY IN METHANOL-WATER MIXED SOLVENT MEDIA A PROJECT WORK SUBMITTED TO THE DEPARTMENT OF CHEMISTRY CENTRAL CAMPUS OF TECHNOLOGY HATTISAR, DHARAN, INSTITUTE OF SCIENCE AND TECHNOLOGY TRIBHUVAN UNIVERSITY, NEPAL, AS A PARTIAL FULFILLMENT OF THE REQUIREMENT FOR THE DEGREE OF BACHELOR OF SCIENCE IN CHEMISTRY BY Shubheksha Dahal EXAMS ROLL NO: 80038 TU REDG NO: 5-2-0008-0054-2013 CENTRAL CAMPUS OF TECHNOLOGY HATTISAR, DHARAN, TRIBHUVAN UNIVERSITY, NEPAL September 2017 i BOARD OF EXAMINER AND CERTIFICATE OF APPROVAL This project work entitled “A Study of Interaction of Cetyltrimethyl Ammonium Bromide with Methyl Red by Conductometry in Methanol-Water Mixed Solvent Media”, by “Shubheksha Dahal” (Roll No.: 80038; T.U. Regd No.: 5-2-0008-0054-2013), under the supervision of “Netra Subedi”, Central Campus of Technology Hattisar, Dharan, Department of Chemistry, Tribhuvan University, Nepal, is hereby submitted for the partial fulfillment of Bachelor of Science (B.Sc.) Degree in Chemistry. This project work had not been submitted in any other university or institution previously and has been approved for the award of degree. …………………………….. Supervisor Mr. Netra Subedi Teaching assistant Department of Chemistry C.C.T., Hattisar, Dharan Tribhuvan University ..………………….......… ….…………………… Internal Examiner External Examiner Department of Chemistry C.C.T., Hattisar, Dharan Tribhuvan University ……………………………… Lalita Shrestha Chairman (Department of Chemistry) Central Campus of Technology, Hattisar T.U., Dharan Sunsari, Nepal Date: … September 2017 ii RECOMMENDATION This is recommending that Shubheksha Dahal(Roll No.: 80038; T.U. Regd No.: 5- 2-0008-0054-2013) has carried out project work entitled “A Study of Interaction of Cetyltrimethyl Ammonium Bromide with Methyl Red by Conductometry in Methanol-Water Mixed Solvent Media” as a partial fulfillment of 4 years Bachelor degree of 4th year in chemistry under my supervision. -

Control of IL J
By Control of IL J. L. Ricard, T. E. See, and W. B. Bollen Oregon State University Incipient Decay Corvallis, Oreg. With Gases in Douglas-fir Poles t-200 THERMOBAROME TER D WOOD AL NE cr 00°A • 0 z METHYL BROMIDE- ALONE - cr 100 cr) METHYL BROMIDE AND WOOD cc Foreword a. In 1962, Messrs. 0. F. Hand and A. F. Wetsch, of the Bonneville Power Administration, began a study of gas diffusion systems for controlling I - decay fungi and insects in Douglas-fir and West- 10 20 30 40 50 ern Red Cedar utility poles. Many fumigants were MINUTES tested in that year, among them Chlorodane, Figure 1. — Evaluation of Warburg apparatus for study Chloropicrin, Ethylene Dibromidc, Vapam, Tc- of methyl bromide absorption by wood at 1.4°C. The break lone, Cresan M, Cyanogas and Methyl Bromide. in the curve for methyl bromide and wood is attributable Fungi and insects were killed by several of the to resetting the manometer. materials tested as confirmed by bio-assay tech- niques. Reported as among the most effective tested were Vapam, Cyanogas, Chloropicrin, and ERVICE LIFE of Douglas-fir poles could be ex- S Methyl Bromide. Following the work by Bonne- tended if they could be penetrated readily by fungicides with long-lasting effects. Incipient ville Power Administration, a cooperative research contract was established with the Forest Research decay is readily detected in poles (16), and the Overholts, Laboratory, Oregon State University, to evaluate common decay fungus, Poria carbonica chemical treatments for arresting decay in poles. is sensitive to fungicides (15). -
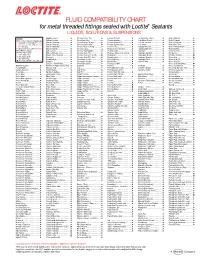
FLUID COMPATIBILITY CHART for Metal Threaded Fittings Sealed with Loctite¨ Sealants LIQUIDS, SOLUTIONS & SUSPENSIONS
FLUID COMPATIBILITY CHART for metal threaded fittings sealed with Loctite® Sealants LIQUIDS, SOLUTIONS & SUSPENSIONS LEGEND: Bagasse Fibers.......................... Chlorobenzene Dry ................... Ferrous Chloride ...................... Ion Exclusion Glycol ................. Nickel Chloride.......................... All Loctite® Anaerobic Sealants are Barium Acetate ........................ Chloroform Dry......................... Ferrous Oxalate......................... Irish Moss Slurry...................... Nickel Cyanide ......................... Compatible Including #242®, 243, Barium Carbonate..................... Chloroformate Methyl............... Ferrous Sulfate10%.................. Iron Ore Taconite ..................... Nickel Fluoborate ..................... 542, 545, 565, 567, 569, 571, 572, Barium Chloride........................ Chlorosulfonic Acid .................. Ferrous Sulfate (Sat)................. Iron Oxide ................................ Nickel Ore Fines ....................... 577, 580, 592 Barium Hydroxide..................... Chrome Acid Cleaning .............. Fertilizer Sol ............................. Isobutyl Alcohol ....................... Nickel Plating Bright ................. † Use Loctite® #270, 271™, 277, 554 Barium Sulfate.......................... Chrome Liquor.......................... Flotation Concentrates.............. Isobutyraldehyde ..................... Nickel Sulfate ........................... Not Recommended Battery Acid .............................. Chrome Plating -

Ammonium Bromide
Safety Data Sheet Ammonium Bromide 1. PRODUCT AND COMPANY IDENTIFICATION Product Name: Ammonium Bromide Synonyms/Generic Names: None SDS Number: 39.00 Product Use: For Educational Use Only Manufacturer: Columbus Chemical Industries, Inc. N4335 Temkin Rd. Columbus, WI. 53925 For More Information Contact: Ward's Science 5100 West Henrietta Rd. PO Box 92912-9012 Rochester, NY 14692 (800) 962-2660 (Monday-Friday 7:30-7:00 Eastern Time) In Case of Emergency Call: CHEMTREC - 800-424-9300 or 703-527-3887 (24 Hours/Day, 7 Days/Week) 2. HAZARDS IDENTIFICATION OSHA Hazards: Target organ effect Target Organs: Central nervous system Signal Words: Warning Pictograms: None GHS Classification: Eye irritation Category 2B Acute toxicity, Oral Category 5 GHS Label Elements, including precautionary statements: Hazard Statements: H303 May be harmful if swallowed. H320 Causes eye irritation. Precautionary Statements: P305+P351+P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do so. Continue rinsing. Potential Health Effects Eyes Causes eye irritation. Inhalation May be harmful if inhaled. Causes respiratory tract irritation. Skin May be harmful if absorbed through skin. Causes skin irritation. Ingestion May be harmful if swallowed. Revised on 06/20/2013 Page 1 of 5 NFPA Ratings HMIS Ratings Health 1 Health 1 Flammability 0 Fire 0 Reactivity 0 Reactivity 0 Specific hazard Not Available Personal B 3. COMPOSITION/INFORMATION ON INGREDIENTS EINECS# / Molecular Component Weight % CAS # Formula ELINCS# Weight Ammonium Bromide 100 12124-97-9 235-183-8 BrNH4 97.94 g/mol 4. FIRST-AID MEASURES Eyes Rinse with plenty of water for at least 15 minutes and seek medical attention if necessary. -

In-Situ Study of Ammonium Bromide Formation for Optimizing Boron Nitride ALD from Bbr3 and NH3
In-situ study of ammonium bromide formation for optimizing boron nitride ALD from BBr3 and NH3 Arnoud J. Onnink, R. Oguzhan Apaydin, Jurriaan Schmitz and Alexey Y. Kovalgin MESA+ Institute for Nanotechnology, University of Twente, the Netherlands. E-mail: [email protected] Boron nitride (BN) has received much attention for a wide range of applications, including in electronics, photonics, and power devices [1]. Among the variety of techniques to deposit thin films of BN, atomic layer deposition (ALD) is an industrially relevant approach due to enabling precise thickness control and excellent conformality [1]. This work studies the deposition of BN from boron tribromide (BBr3) and ammonia (NH3) by in-situ real-time monitoring of the reactive gases and reaction byproducts, using Fourier Transform infrared (FTIR) spectroscopy and spectroscopic ellipsometry (SE) as schematically shown in figure 1a. Depending on the chosen conditions, the deposition of BN varies between ALD and pulsed chemical vapor deposition (CVD). Figures 1b-c show a layer of particles deposited on a cold (293 K) substrate near the exhaust, identified as ammonium bromide (NH4Br) by the FTIR reflectance spectrum. Such salt is a known byproduct in CVD from NH3 and halide precursor [2], forming from NH3 and the gaseous hydrogen halide byproduct (here, HBr) and spreading through the reactor by sublimation, dissociation [3] to HBr + NH3, and redeposition. To optimize an ALD recipe, the precursor-pulse and purge durations are typically increased until the growth per cycle (GPC) saturates. This work explores a new method to optimize BN ALD based on in-situ monitoring by FTIR and SE of the precursors and reaction byproducts. -

Ammonium Bromide MSDS # 39.00
Material Safety Data Sheet Page 1 of 2 Ammonium Bromide MSDS # 39.00 Section 1: Product and Company Identification Ammonium Bromide Synonyms/General Names: N/A Product Use: For educational use only Manufacturer: Columbus Chemical Industries, Inc., Columbus, WI 53925. 24 Hour Emergency Information Telephone Numbers CHEMTREC (USA): 800-424-9300 CANUTEC (Canada): 613-424-6666 ScholAR Chemistry; 5100 W. Henrietta Rd, Rochester, NY 14586; (866) 260-0501; www.Scholarchemistry.com Section 2: Hazards Identification White crystalline powder; no odor. HMIS (0 to 4) Health 1 CAUTION! Slightly toxic by ingestion. Fire Hazard 0 Target organs: Central nervous system. Reactivity 0 This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Section 3: Composition / Information on Ingredients Ammonium Bromide, (12124-97-9), 100% Section 4: First Aid Measures Always seek professional medical attention after first aid measures are provided. Eyes: Immediately flush eyes with excess water for 15 minutes, lifting lower and upper eyelids occasionally. Skin: Immediately flush skin with excess water for 15 minutes while removing contaminated clothing. Ingestion: Call Poison Control immediately. Rinse mouth with cold water. Give victim 1-2 cups of water or milk to drink. Induce vomiting immediately. Inhalation: Remove to fresh air. If not breathing, give artificial respiration. Section 5: Fire Fighting Measures When heated to decomposition, emits acrid fumes of NOx, ammonia and hydrogen bromide. 0 Protective equipment and precautions for firefighters: Use foam or dry chemical to extinguish fire. 1 0 Firefighters should wear full fire fighting turn-out gear and respiratory protection (SCBA). Cool container with water spray. -

Semiclathrate Hydrate Phase Equilibria for CO2 in the Presence of Tetra-N-Butyl Ammonium Halide (Bromide, Chloride, Or Fluoride)
See discussions, stats, and author profiles for this publication at: http://www.researchgate.net/publication/228478994 Semiclathrate Hydrate Phase Equilibria for CO2 in the Presence of Tetra-n-butyl Ammonium Halide (Bromide, Chloride, or Fluoride) ARTICLE in JOURNAL OF CHEMICAL & ENGINEERING DATA · SEPTEMBER 2010 Impact Factor: 2.04 · DOI: 10.1021/je100059h CITATIONS READS 74 93 5 AUTHORS, INCLUDING: Shifeng Li Shuanshi Fan Shenyang University South China University of Technology 18 PUBLICATIONS 256 CITATIONS 140 PUBLICATIONS 7,618 CITATIONS SEE PROFILE SEE PROFILE Xuemei Lang Yanhong Wang South China University of Technology Northeast Agricultural University 28 PUBLICATIONS 508 CITATIONS 27 PUBLICATIONS 330 CITATIONS SEE PROFILE SEE PROFILE Available from: Shuanshi Fan Retrieved on: 07 December 2015 J. Chem. Eng. Data XXXX, xxx, 000 A Semiclathrate Hydrate Phase Equilibria for CO2 in the Presence of Tetra-n-butyl Ammonium Halide (Bromide, Chloride, or Fluoride) Shifeng Li,†,‡ Shuanshi Fan,*,‡ Jingqu Wang,† Xuemei Lang,‡ and Yanhong Wang‡ State Key Laboratory of Fine Chemicals, School of Chemical Engineering, Dalian University of Technology, Dalian 116012, China, and Key Laboratory of Enhanced Heat Transfer and Energy Conversation, Ministry of Education, South China University of Technology, Guangzhou 510640, China Phase equilibrium data for the semiclathrate hydrates formed in three three-component systems, the CO2 + tetra-n-butyl ammonium bromide (TBAB) + water system, the CO2 + tetra-n-butyl ammonium chloride (TBAC) + water system, and the CO2 + tetra-n-butyl ammonium fluoride (TBAF) + water system, were measured in the pressure range of (0.40 to 3.77 MPa) and temperature range of (280.2 to 293.5 K) at (2.93 · 10-3 and 6.17 · 10-3) mole fraction of tetra-n-butyl ammonium halide. -

Ammonium Bromide
CLH REPORT FOR AMMONIUM BROMIDE CLH report Proposal for Harmonised Classification and Labelling Based on Regulation (EC) No 1272/2008 (CLP Regulation), Annex VI, Part 2 International Chemical Identification: Ammonium bromide EC Number: 235-183-8 CAS Number: 12124-97-9 Index Number: - Contact details for dossier submitter: Swedish Chemicals Agency Esplanaden 3a, P.O Box 2 SE-172 13 Sundbyberg, Sweden [email protected] +46 8 519 41 100 Version number: 2 Date: 190222 CLH REPORT FOR AMMONIUM BROMIDE CONTENTS 1 IDENTITY OF THE SUBSTANCE ...................................................................................................................... 1 1.1 NAME AND OTHER IDENTIFIERS OF THE SUBSTANCE .............................................................................................. 1 1.2 COMPOSITION OF THE SUBSTANCE ........................................................................................................................ 1 2 PROPOSED HARMONISED CLASSIFICATION AND LABELLING ........................................................... 3 2.1 PROPOSED HARMONISED CLASSIFICATION AND LABELLING ACCORDING TO THE CLP CRITERIA ........................... 3 3 HISTORY OF THE PREVIOUS CLASSIFICATION AND LABELLING ..................................................... 4 4 JUSTIFICATION THAT ACTION IS NEEDED AT COMMUNITY LEVEL ................................................ 5 5 IDENTIFIED USES ............................................................................................................................................... -

UNION CHRISTIAN COLLEGE Aluva- 683 102 (Affiliated to Mahatma Gandhi University, Kottayam) Ernakulam Dt
Post Box: 5 UNION CHRISTIAN COLLEGE Aluva- 683 102 (Affiliated to Mahatma Gandhi University, Kottayam) Ernakulam Dt. Kerala Fax: 91-484- 2607534 Accredited by NAAC with “A” Grade, Cycle-4 91-484-2606033, 2609194 Email: [email protected] LIMITED TENDER NOTICE No. Department: Chemistry Sealed tenders are invited for the supply of the following Items: CHEMICALS ACETANILIDE ACETONE ACETONE COMML ACETOPHENONE ACETIC ACID GLACIAL ACID HYDROCHLORIC PURE ACID HYDROCHLORIC COMML ACID NITRIC COMML ACID NITRIC PURE ACID ORTHO- PHOSPHORIC ACID SULPHURIC COMML ACID SULPHURIC PURE AGAR AGAR ALCOHOL AMYL ISO – AMYL ALCOHOL ALUMINA (NEUTRAL) ALUMINIUM CHLORIDE ALUMINIUM NITRATE ALUMINIUM SULPHATE AMINO NAPHTHALENE- ALPHA AMINO PHENOL AMMONIA SOLUTION ER / AR AMMONIUM CARBONATE AMMONIUM ACETATE AMMONIUM BROMIDE AMMONIUM BORATE AMMONIUM CERIC SULPHATE AMMONIUM CERIC NITRATE AMMONIUM CHLORIDE AR AMMONIUM CHLORIDE PURE AMMONIUM CHLORIDE COMML AMMONIUM FORMATE AMMONIUM FLURIDE AMMONIUM NITRITE AMMONIUM NITRATE AMMONIUM NICKEL(II) SULPHATE AMMONIUM OXALATE AMMONIUM PURPURATE (MUREXIDE) AMMONIUM SULPHATE AMMONIUM SULPHIDE AMMONIUM IRON SULPHATE AMMONIUM FERROUS SULFATE AMMONIUM MOLYBDATE AMMONIUM IRON (II) SULPHATE 2-AMINO PYRIDINE Mohr's Salt AMMONIUM THIO CYNATE AMMONIUM THIOSULPHATE ANILINE ANISOLE PARA ANISALDEHYDE ANTHRACENE ANTHRANILIC ACID BARIUM CHLORIDE AR BARIUM CHLORIDE PURE BARIUM NITRATE BENZALDEHYDE BENZENE BENZOIC ACID BENZOIN BENZOPHENONE BENZIL BENZYL ALCOHOL BROMINE BIPHENYLE CALCIUM CHLORIDE (ANHYDROUS) CALCIUM FLURIDE CALCIUM HYDROXIDE CALCIUM -

Chemical Compatibility Storage Group
CHEMICAL SEGREGATION Chemicals are to be segregated into 11 different categories depending on the compatibility of that chemical with other chemicals The Storage Groups are as follows: Group A – Compatible Organic Acids Group B – Compatible Pyrophoric & Water Reactive Materials Group C – Compatible Inorganic Bases Group D – Compatible Organic Acids Group E – Compatible Oxidizers including Peroxides Group F– Compatible Inorganic Acids not including Oxidizers or Combustible Group G – Not Intrinsically Reactive or Flammable or Combustible Group J* – Poison Compressed Gases Group K* – Compatible Explosive or other highly Unstable Material Group L – Non-Reactive Flammable and Combustible, including solvents Group X* – Incompatible with ALL other storage groups The following is a list of chemicals and their compatibility storage codes. This is not a complete list of chemicals, but is provided to give examples of each storage group: Storage Group A 94‐75‐7 2,4‐D (2,4‐Dichlorophenoxyacetic acid) 94‐82‐6 2,4‐DB 609-99-4 3,5-Dinitrosalicylic acid 64‐19‐7 Acetic acid (Flammable liquid @ 102°F avoid alcohols, Amines, ox agents see SDS) 631-61-8 Acetic acid, Ammonium salt (Ammonium acetate) 108-24-7 Acetic anhydride (Flammable liquid @102°F avoid alcohols see SDS) 79‐10‐7 Acrylic acid Peroxide Former 65‐85‐0 Benzoic acid 98‐07‐7 Benzotrichloride 98‐88‐4 Benzoyl chloride 107-92-6 Butyric Acid 115‐28‐6 Chlorendic acid 79‐11‐8 Chloroacetic acid 627‐11‐2 Chloroethyl chloroformate 77‐92‐9 Citric acid 5949-29-1 Citric acid monohydrate 57-00-1 Creatine 20624-25-3 -

Ammonium Bromide 1
MSDS Number: A5664 * * * * * Effective Date: 09/02/09 * * * * * Supercedes: 03/14/07 AMMONIUM BROMIDE 1. Product Identification Synonyms: Hydrobromic acid monoammoniate CAS No.: 12124-97-9 Molecular Weight: 97.95 Chemical Formula: NH4Br Product Codes: J.T. Baker: 0636 Mallinckrodt: 0424 2. Composition/Information on Ingredients Ingredient CAS No Percent Hazardous --------------------------------------- ------------ ------------ --------- Ammonium Bromide /(NH4)Br/ 12124-97-9 100% Yes 3. Hazards Identification Emergency Overview -------------------------- WARNING! HARMFUL IF SWALLOWED OR INHALED. AFFECTS CENTRAL NERVOUS SYSTEM, BRAIN AND EYES. MAY CAUSE IRRITATION TO SKIN, EYES, AND RESPIRATORY TRACT. SAF-T-DATA(tm) Ratings (Provided here for your convenience) ----------------------------------------------------------------------------------------------------------- Health Rating: 2 - Moderate (Life) Flammability Rating: 0 - None Reactivity Rating: 2 - Moderate Contact Rating: 2 - Moderate Lab Protective Equip: GOGGLES & SHIELD; LAB COAT & APRON; VENT HOOD; PROPER GLOVES Storage Color Code: Green (General Storage) ----------------------------------------------------------------------------------------------------------- Potential Health Effects ---------------------------------- Inhalation: Dust may cause irritation to the respiratory tract. Symptoms may include coughing, sore throat, and shortness of breath. Ingestion: May cause nausea, vomiting and abdominal pain. Ingestions are usually promptly rejected by vomiting, but sufficient absorption