Lemtrada (Alemtuzumab)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alemtuzumab Comparison with Rituximab and Leukemia Whole
Mechanism of Action of Type II, Glycoengineered, Anti-CD20 Monoclonal Antibody GA101 in B-Chronic Lymphocytic Leukemia Whole Blood Assays in This information is current as Comparison with Rituximab and of September 27, 2021. Alemtuzumab Luca Bologna, Elisa Gotti, Massimiliano Manganini, Alessandro Rambaldi, Tamara Intermesoli, Martino Introna and Josée Golay Downloaded from J Immunol 2011; 186:3762-3769; Prepublished online 4 February 2011; doi: 10.4049/jimmunol.1000303 http://www.jimmunol.org/content/186/6/3762 http://www.jimmunol.org/ Supplementary http://www.jimmunol.org/content/suppl/2011/02/04/jimmunol.100030 Material 3.DC1 References This article cites 44 articles, 24 of which you can access for free at: http://www.jimmunol.org/content/186/6/3762.full#ref-list-1 by guest on September 27, 2021 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2011 by The American -

Activity of Rituximab and Ofatumumab Against Mantle
ACTIVITY OF RITUXIMAB AND OFATUMUMAB AGAINST MANTLE CELL LYMPHOMA(MCL) IN VITRO IN MCL CELL LINES BY COMPLEMENT DEPENDENT CYTOTOXICITY (CDC)AND ANTIBODY-DEPENDENT CELL MEDIATED CYTOTOXICITY ASSAYS(ADCC) Dr. Gopichand Pendurti M.B.B.S Mentor: Dr. Francisco J. Hernandez-Ilizaliturri MD Overview of presentation •Introduction to mantle cell lymphoma. •Concept of minimal residual disease. •Anti CD 20 antibodies. •51Cr release assays. •Flow cytometry on cell lines. •Results. •Future. MANTLE CELL LYMPHOMA •Mantle cell lymphoma is characterized by abnormal proliferation of mature B lymphocytes derived from naïve B cells. •Constitutes about 5% of all patients with Non Hodgkin's lymphoma. •Predominantly in males with M:F ratio 2.7:1 with onset at advanced age (median age 60yrs). •It is an aggressive lymphoma with median survival of patients being 3-4 years. •Often presents as stage III-IV with lymphadenopathy, hepatosplenomegaly, gastrointestinal involvement, peripheral blood involvement. Pedro Jares, Dolors Colomer and Elias Campo Genetic and molecular pathogenesis of mantle cell lymphoma: perspectives for new targeted therapeutics Nature revision of cancer 2007 October:7(10):750-62 •Genetic hallmark is t(11:14)(q13:q32) translocation leading to over expression of cyclin D1 which has one of the important pathogenetic role in deregulating the cell cycle. •Other pathogentic mechanisms include molecular and chromosomal alterations that Target proteins that regulate the cell cycle and senecense (BMI1,INK4a,ARF,CDK4 AND RB1). Interfere with cellular -

Glatiramer Acetate
Clinical Policy: Glatiramer Acetate (Copaxone, Glatopa) Reference Number: CP.PHAR.252 Effective Date: 09.01.16 Last Review Date: 05.20 Coding Implications Line of Business: Commercial, HIM, Medicaid Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description Glatiramer acetate (Copaxone®, Glatopa®) is a polypeptide. FDA Approved Indication(s) Copaxone and Glatopa are indicated for the treatment of patients with relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults. Policy/Criteria Provider must submit documentation (such as office chart notes, lab results or other clinical information) supporting that member has met all approval criteria. It is the policy of health plans affiliated with Centene Corporation® that Copaxone and Glatopa are medically necessary when the following criteria are met: I. Initial Approval Criteria A. Multiple Sclerosis (must meet all): 1. Diagnosis of one of the following (a, b, or c): a. Clinically isolated syndrome; b. Relapsing-remitting MS; c. Secondary progressive MS; 2. Prescribed by or in consultation with a neurologist; 3. Age ≥ 18 years; 4. If request is for brand Copaxone, member has experienced clinically significant adverse effects to generic glatiramer (including Glatopa) or has contraindication(s) to its excipients; 5. Glatiramer is not prescribed concurrently with other disease modifying therapies for MS (see Appendix D); 6. Dose does not exceed 20 mg per day (1 prefilled syringe per day) or 40 mg three times per week (3 prefilled syringes per week). Approval duration: Medicaid/HIM – 6 months Commercial – 6 months or to the member’s renewal date, whichever is longer B. -

The Change of Fingolimod Patient Profiles Over Time
Journal of Personalized Medicine Article The Change of Fingolimod Patient Profiles over Time: A Descriptive Analysis of Two Non-Interventional Studies PANGAEA and PANGAEA 2.0 Tjalf Ziemssen 1,* and Ulf Schulze-Topphoff 2 1 Zentrum für Klinische Neurowissenschaften, Universitätsklinikum Carl Gustav Carus, D-01307 Dresden, Germany 2 Novartis Pharma GmbH, D-90429 Nuremberg, Germany; [email protected] * Correspondence: [email protected] Abstract: (1) Background: Fingolimod (Gilenya®) was the first oral treatment for patients with relapsing-remitting multiple sclerosis (RRMS). Since its approval, the treatment landscape has changed enormously. (2) Methods: Data of PANGAEA and PANGAEA 2.0, two German real-world studies, were descriptively analysed for possible evolution of patient profiles and treatment behavior. Both are prospective, multi-center, non-interventional, long-term studies on fingolimod use in RRMS in real life. Data of 4229 PANGAEA patients (recruited 2011–2013) and 2441 PANGAEA 2.0 patients (recruited 2015–2018) were available. Baseline data included demographics, RRMS characteristics and disease severity. (3) Results: The mean age of PANGAEA and PANGAEA 2.0 patients was similar (38.8 vs. 39.2 years). Patients in PANGAEA 2.0 had shorter disease duration (7.1 vs. 8.2 years) and fewer relapses in the year before baseline (1.2 vs. 1.6). Disease severity at baseline estimated by Citation: Ziemssen, T.; EDSS and SDMT was lower in PANGAEA 2.0 patients compared to PANGAEA (EDSS difference Schulze-Topphoff, U. The Change of 1.0 points; SDMT difference 3.3 points). (4) Conclusions: The results hint at an influence of changes in Fingolimod Patient Profiles over the treatment guidelines and the label on fingolimod patients profiles over time. -

Alemtuzumab Comparison with Rituximab and Leukemia Whole
Mechanism of Action of Type II, Glycoengineered, Anti-CD20 Monoclonal Antibody GA101 in B-Chronic Lymphocytic Leukemia Whole Blood Assays in This information is current as Comparison with Rituximab and of September 29, 2021. Alemtuzumab Luca Bologna, Elisa Gotti, Massimiliano Manganini, Alessandro Rambaldi, Tamara Intermesoli, Martino Introna and Josée Golay Downloaded from J Immunol 2011; 186:3762-3769; Prepublished online 4 February 2011; doi: 10.4049/jimmunol.1000303 http://www.jimmunol.org/content/186/6/3762 http://www.jimmunol.org/ Supplementary http://www.jimmunol.org/content/suppl/2011/02/04/jimmunol.100030 Material 3.DC1 References This article cites 44 articles, 24 of which you can access for free at: http://www.jimmunol.org/content/186/6/3762.full#ref-list-1 by guest on September 29, 2021 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2011 by The American -

Medical Review(S) Clinical Review
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 22-527 MEDICAL REVIEW(S) CLINICAL REVIEW Application Type NDA Application Number 22-527 Priority or Standard P Submit Date 12-21-2009 Received Date 12-21-2009 PDUFA Goal Date 9-21-2010 Division / Office FDA/ODE1 Reviewer Name Heather D. Fitter Review Completion Date August 26, 2010 Established Name Fingolimod (Proposed) Trade Name Gilenya Therapeutic Class S1P receptor modulator Applicant Novartis Formulation(s) Oral tablets Dosing Regimen 0.5 mg Indication(s) To reduce the frequency of relapses and delay the progression of disability in relapsing MS Intended Population(s) Relapsing Multiple Sclerosis Template Version: March 6, 2009 Clinical Review Heather D. Fitter, M.D. NDA 22-527 Fingolimod Table of Contents 1 RECOMMENDATIONS/RISK BENEFIT ASSESSMENT......................................... 7 1.1 Recommendation on Regulatory Action ............................................................. 7 1.2 Risk Benefit Assessment.................................................................................... 8 2 INTRODUCTION AND REGULATORY BACKGROUND ........................................ 8 2.1 Product Information ............................................................................................ 9 2.2 Table of Currently Available Treatments for the Proposed Indication................. 9 2.3 Availability of Proposed Active Ingredient in the United States ........................ 12 2.4 Important Safety Issues with Consideration to Related Drugs......................... -

Clinical Policy: Interferon Beta-1B (Betaseron, Extavia)
Clinical Policy: Interferon Beta-1b (Betaseron, Extavia) Reference Number: CP.CPA.331 Effective Date: 06.01.18 Last Review Date: 05.19 Coding Implications Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description Interferon beta-1b (Betaseron®, Extavia®) is an amino acid glycoprotein. FDA Approved Indication(s) Betaseron and Extavia are indicated for the treatment of patients with relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults. Policy/Criteria Provider must submit documentation (such as office chart notes, lab results or other clinical information) supporting that member has met all approval criteria. It is the policy of health plans affiliated with Centene Corporation® that Betaseron and Extavia are medically necessary when the following criteria are met: I. Initial Approval Criteria A. Multiple Sclerosis (must meet all): 1. Diagnosis of one of the following (a, b, or c): a. Clinically isolated syndrome (CIS); b. Relapsing-remitting MS (RRMS); c. Secondary progressive MS (SPMS); 2. Prescribed by or in consultation with a neurologist; 3. Age ≥ 12 years; 4. For Extavia requests, member meets one of the following (a or b): a. If RRMS and age ≥ 18 years: Failure of two of the following at up to maximally indicated doses, unless contraindicated or clinically significant adverse effects are experienced: Aubagio®, Tecfidera®, Gilenya™, Avonex®, Betaseron®, Plegridy®, glatiramer, Copaxone®, Glatopa®, or Rebif®; b. If CIS or SPMS: Failure of two of the following at up to maximally indicated doses, unless contraindicated or clinically significant adverse effects are experienced: Avonex, Betaseron, Plegridy, or Rebif; *Prior authorization is required for all disease modifying therapies for MS 5. -

Comparative Clinical and Cost-Effectiveness of Drug
Table 46: Univariate Sensitivity Analysis Regarding Cost of Relapse Cost per relapse = $1,405 Grima et al.74 ICUR versus Treatment Total Cost Total QALYs glatiramer Sequential ICUR acetate Glatiramer acetate $288,507 11.272 Ref Ref (Copaxone) Interferon beta-1b $299,930 11.376 $109,519 $109,519 (Extavia) Dimethyl fumarate $332,772 11.442 $261,092 $503,474 (Tecfidera) Natalizumab (Tysabri) $459,081 11.580 $554,068 $913,177 Dominated treatments Dominated by interferon Interferon beta-1b $313,300 11.376 $237,687 beta-1b 250 mcg (Betaseron) (Extavia) Dominated by interferon Interferon beta-1a 22 beta-1b 250 mcg $316,087 11.187 Dominated mcg (Rebif) (Extavia) and glatiramer acetate Dominated by interferon Interferon beta-1a beta-1b 250 mcg $319,267 11.167 Dominated (Avonex) (Extavia) and glatiramer acetate Dominated by interferon beta-1b 250 mcg Interferon beta-1a 44 $344,575 11.262 Dominated (Extavia), glatiramer mcg (Rebif) acetate and dimethyl fumarate Dominated by dimethyl Fingolimod (Gilenya) $389,409 11.422 $673,157 fumarate Cost per relapse = $6,402 Karampampa et al.75 Glatiramer acetate $311,927 11.272 Ref Ref (Copaxone) Interferon beta-1b $323,995 11.376 $115,694 $115,694 (Extavia) Dimethyl fumarate $353,243 11.442 $243,695 $448,383 (Tecfidera) Natalizumab (Tysabri) $475,615 11.580 $531,700 $884,714 Dominated treatments Dominated by interferon Interferon beta-1b $337,364 11.395 $243,863 beta-1b 250 mcg (Betaseron) (Extavia) Dominated by interferon Interferon beta-1a 22 beta-1b 250 mcg $340,051 11.190 Dominated mcg (Rebif) (Extavia) -

Refreshing the Biologic Pipeline 2020
news feature Credit: Science Lab / Alamy Stock Photo Refreshing the biologic pipeline 2020 In the absence of face-to-face meetings, FDA and industry implemented regulatory workarounds to maintain drug and biologics approvals. These could be here to stay. John Hodgson OVID-19 might have been expected since 1996) — a small miracle in itself “COVID-19 confronted us with the need to severely impair drug approvals (Fig. 1 and Table 1). to better triage sponsors’ questions,” says Cin 2020. In the event, however, To the usual crop of rare disease and Peter Marks, the director of the Center for industry and regulators delivered a small genetic-niche cancer treatments, 2020 Biologics Evaluation and Research (CBER) miracle. They found workarounds and also added a chimeric antigen receptor at the FDA. “That was perhaps the single surrogate methods of engagement. Starting (CAR)-T cell therapy with a cleaner biggest takeaway from the pandemic related in January 2020, when the outbreak veered manufacturing process and the first to product applications.” Marks says that it westward, the number of face-to face approved blockbuster indication for a became very apparent with some COVID- meetings declined rapidly; by March, small-interfering RNA (siRNA) — the 19-related files that resolving a single they were replaced by Webex and Teams. European Medicines Agency’s (EMA) issue can help a sponsor enormously and (Secure Zoom meeting are to be added registration of the RNA interference accelerate the development cycle. Before this year.) And remarkably, by 31 December, (RNAi) therapy Leqvio (inclisiran) for COVID-19, it was conceivable that a small the US Food and Drug Administration cardiovascular disease. -

Horizon Scanning Status Report June 2019
Statement of Funding and Purpose This report incorporates data collected during implementation of the Patient-Centered Outcomes Research Institute (PCORI) Health Care Horizon Scanning System, operated by ECRI Institute under contract to PCORI, Washington, DC (Contract No. MSA-HORIZSCAN-ECRI-ENG- 2018.7.12). The findings and conclusions in this document are those of the authors, who are responsible for its content. No statement in this report should be construed as an official position of PCORI. An intervention that potentially meets inclusion criteria might not appear in this report simply because the horizon scanning system has not yet detected it or it does not yet meet inclusion criteria outlined in the PCORI Health Care Horizon Scanning System: Horizon Scanning Protocol and Operations Manual. Inclusion or absence of interventions in the horizon scanning reports will change over time as new information is collected; therefore, inclusion or absence should not be construed as either an endorsement or rejection of specific interventions. A representative from PCORI served as a contracting officer’s technical representative and provided input during the implementation of the horizon scanning system. PCORI does not directly participate in horizon scanning or assessing leads or topics and did not provide opinions regarding potential impact of interventions. Financial Disclosure Statement None of the individuals compiling this information have any affiliations or financial involvement that conflicts with the material presented in this report. Public Domain Notice This document is in the public domain and may be used and reprinted without special permission. Citation of the source is appreciated. All statements, findings, and conclusions in this publication are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI) or its Board of Governors. -
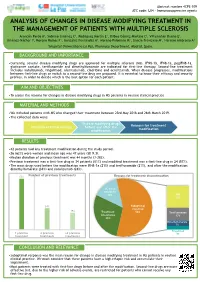
Analysis of Changes in Disease Modifying Treatment in The
Abstract number 4CPS-109 ATC code: L04 - Immunosuppressive agents ANALYSIS OF CHANGES IN DISEASE MODIFYING TREATMENT IN THE MANAGEMENT OF PATIENTS WITH MULTIPLE SCLEROSIS Arancón Pardo A1, Sobrino Jiménez C1, Rodríguez Martín E1, Bilbao Gómez-Martino C1, Villamañán Bueno E1, Jiménez-Nácher I1, Moreno Ramos F1, González Fernández A1, Moreno Palomino M1, García-Trevijano M1, Herrero Ambrosio A1 1Hospital Universitario La Paz, Pharmacy Department, Madrid, Spain. BACKGROUND AND IMPORTANCE •Currently, several disease modifying drugs are approved for multiple sclerosis (MS). IFNβ-1b, IFNβ-1a, pegIFNβ-1a, glatiramer acetate, teriflunomide and dimethylfumarate are indicated for first-line therapy. Second-line treatment includes natalizumab, fingolimod, alemtuzumab, cladribine and ocrelizumab. When disease progresses, modifications between first-line drugs or switch to a second-line drug are proposed. It is essential to know their efficacy and security profiles, in order to decide which is the best option for each patient. AIM AND OBJECTIVES •To assess the reasons for changes in disease modifying drugs in MS patients in routine clinical practice. MATERIAL AND METHODS •We included patients with MS who changed their treatment between 23rd May 2018 and 26th March 2019. •The collected data were: Disease modifying drugs Reasons for treatment Duration of initial therapy before and after the modification modification RESULTS •42 patients had any treatment modification during the study period. •26 (62%) were women and mean age was 47 years (SD 9.3). •Median duration of previous treatment was 44 months (3-282). •Previous treatment was a first-line drug in 34 patients (81%) and modified treatment was a first-line drug in 24 (57%). -

Patient Focused Disease State and Assistance Programs
Patient Focused Disease State and Assistance Programs Medication Medication Toll-free Brand (Generic) Website number Additional Resources Allergy/Asthma Xolair (omalizumab) xolair.com 1-866-4-XOLAIR lung.org Cardiovascular Pradaxa (dabigatran) pradaxa.com 877-481-5332 heart.org Praluent (alirocumab) praluent.com 844-PRALUENT thefhfoundation.org Repatha (evolocumab) repatha.com 844-REPATHA Tikosyn (dofetilide) tikosyn.com 800-879-3477 Crohn’s Disease Cimzia (certolizumab pegol) cimzia.com 866-4-CIMZIA crohnsandcolitis.com Humira (adalimumab) humira.com 800-4-HUMIRA crohnsforum.com Stelara (ustekinumab) stelarainfo.com 877-STELARA Dermatology Cosentyx (secukinumab) cosentyx.com 844-COSENTYX psoriasis.org Dupixent (dupilumab) dupixent.com 844-DUPIXENT nationaleczema.org Enbrel (etanercept) enbrel.com 888-4-ENBREL Humira (adalimumab) humira.com 800-4-HUMIRA Otezla (apremilast) otezla.com 844-4-OTEZLA Stelara (ustekinumab) stelarainfo.com 877-STELARA Taltz (ixekizumab) taltz.com 800-545-5979 Hematology Aranesp (darbepoetin alfa) aranesp.com 805-447-1000 chemocare.com Granix (filgrastim) granixrx.com 888-4-TEVARX hematology.org Jadenu (deferasirox) jadenu.com 888-282-7630 Neulasta (pegfilgrastim) neulasta.com 800-77-AMGEN Neupogen (filgrastim) neupogen.com 800-77-AMGEN Nivestym (filgrastim) nivestym.com 800-879-3477 Zarxio (filgrastim) zarxio.com 800-525-8747 Zytiga (abiraterone) zytiga.com 800-JANSSEN Hepatitis B Baraclude (entecavir) baraclude.com 800-321-1335 cdc.gov Viread (tenofovir disoproxil viread.com 800-GILEAD-5 hepb.org fumarate)