West Midlands North
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Norton Aluminium Residents Liaison Committee- Meeting Minutes Norton Canes Community Centre, Brownhills Road, Norton Canes 16Th June 2016 1800-2030
Norton Aluminium Residents Liaison Committee- Meeting Minutes Norton Canes Community Centre, Brownhills Road, Norton Canes 16th June 2016 1800-2030 List of Attendees Cllr John Bernard- Chairman of Liaison Committee & Norton Canes Parish Councilor. CCDC Mike Walker, Environmental Protection Manager Chris Richardson, Scientific Officer Norton Aluminium Trevor Bird Foundry Manager, Andrew Street, Environmental Manager, Wayne Harrison Production Manager Paul Clews, Maintenance Manager. Residents Rodney Brown- Vice Chairman Lenard Sharratt Robert Oddy Stephen Hawkins Paul Sanders Meeting opened Cllr Bernard opened the meeting and introduced himself as Chairman of the Norton Aluminum Liaison Committee. Confirmed Mr Rodney Brown was still Vice Chairman. Introductions All parties introduced themselves. Terms of reference- Purpose of meeting Cllr Bernard advised purpose of meeting was to continue to provide a forum at which issues relating to the operation of the site and any concerns of local residents, Councillors and Council Officers can be addressed. It was agreed by the committee that three meetings per year will continue to be held. Option to hold extraordinary meeting if required. Terms of reference agreed by all parties. Presentation by Norton Aluminium Presentation by Mr Wayne Harrison Foundry Manager providing overview of the process at Norton Aluminium. Detailed types of material the company melts (dross, engine blocks, pucks, coppers silicon, aluminium wheels etc) Provided an overview of the furnaces (rotaries & holding furnaces), extraction, launders and finished products. An example charge card passed around the group detailing how product information is required. Questions RO asked what happens as regards fumes & extraction within the plant. TB provided an overview of the DISA plant bag filtration system and how it operates. -
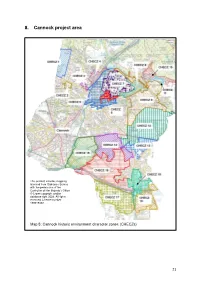
8. Cannock Project Area
8. Cannock project area This product includes mapping licensed from Ordnance Survey with the permission of the Controller of Her Majesty’s Office © Crown copyright and/or database right 2009. All rights reserved. Licence number 100019422. Map 5: Cannock historic environment character zones (CHECZs) 21 8.1 CHECZ 1 – West of Pye Green 8.1.1 Summary on the historic environment The zone comprises a very large field, as depicted on map 6, which was created during the late 20th century through the removal of earlier internal boundaries. The field system was originally created as planned enclosure following an Act of Parliament to enclose (1868). Prior to this period the landscape had been dominated by heath land which had formed part of Cannock Chase. The nursery and its surrounding boundary also post date the Second World War. This product includes mapping data licensed from Ordnance Survey © Crown copyright and / or database right (2009). Licence no. 100019422 Map 6: The known heritage assets (sites referred to in the text are labelled). Of particular significance is the remains of a bank which follows the western boundary of the zone38. This feature is contiguous with the parish boundary between Huntington and Cannock. It is therefore possible that this bank was constructed in 38 Staffordshire HER: PRN 01039 22 the medieval or post medieval period to physically demarcate the parish bounds or the extent of the medieval manor of Cannock. 8.1.2 Heritage Assets Summary Table Survival The zone has seen moderate disturbance 2 from agricultural practices, although the earthwork bank was surviving in 2000. -

Cannock Chase District Housing Development Capacity Study 2018–38 March 2021
CANNOCK CHASE DISTRICT HOUSING DEVELOPMENT CAPACITY STUDY 2018–38 MARCH 2021 Planning Policy Team Cannock Chase District Council V7 10/03/21 0 CANNOCK CHASE DISTRICT – DEVELOPMENT CAPACITY STUDY (HOUSING) CONTENTS 1. INTRODUCTION 2. NATIONAL POLICY CONTEXT 3. REQUIRED CAPACITY OF LAND FOR HOUSING DEVELOPMENT (2018-38) 3.1 Assessed Housing Need (2018-38) 3.2 Provision for the Needs of Neighbouring Areas under Duty to Co-operate 4. CONFIRMED HOUSING LAND SUPPLY CAPACITY (2018-38) 4.1 SHLAA Housing Completions (2018-20) 4.2 SHLAA Deliverable Sites 4.3 SHLAA Developable Sites (Adopted Local Plan Period to 2028) 4.4 Total Confirmed Housing Land Supply Capacity (2018-38) 5. POTENTIAL HOUSING LAND SUPPLY CAPACITY (2018-38) 5.1 SHLAA Developable Sites (Post Adopted Local Plan Period to 2028) 5.2 SHLAA ‘Restricted and Excluded’ Sites 5.3 Employment Land Availability Assessment ‘Restricted and Excluded’ Sites 5.4 Total Potential Housing Supply Capacity (2018-38) 6. OTHER POTENTIAL HOUSING LAND SUPPLY OPTIONS 6.1 SHLAA Green Belt and/or Green Space Network 6.2 ELAA Green Belt and Green Space Network 6.3 Restricted and Excluded Sites in Alternative Uses 6.4 Neighbourhood Plans 6.5 Cannock Chase Open Space Review 6.6 Housing Estates and Redevelopment 6.7 Public Sector Surplus Land 6.8 Reallocation of Existing Employment Land 6.9 Review of Brownfield Land Register and the National Land Use Database 6.10 Regeneration Sites Promoted for Residential Development 6.11 Sites where Planning Applications were Refused or Withdrawn (2018-20) 6.12 Contributions from Self Build Housing 6.13 Potential Contributions from new Permitted Development Rights 6.14 Contributions from Exception Sites 6.15 Reviewing Density Assumptions 6.16 Additional Potential Sites Identified During Study Process 7. -

Norton Canes Staffordshire Case Study: Residential Sector
Norton Canes Staffordshire Case Study: Residential Sector The Project In 2006 Richborough Estates negotiated an agreement to purchase a former greyhound racing track in Norton Canes, subject to securing residential planning permission. The derelict track and an existing garden centre was situated in the Green Belt, totalling approximately 21 acres. The Approach • The site was identified by Cannock Chase District Council as a preferred location for housing and was promoted through the Core Strategy process • A public consultation with the local community was held to address their concerns, centered around the impact of the development upon the Green Belt, local infrastructure and traffic congestion • Improvements to the local community centre and the provision of alternative grazing land was proposed following consultation with the Parish Council • The site had ecological issues which had to be addressed within the confines of the Cannock Chase Special Area of Conservation The Result • Unanimous approval for up to 130 residential units by Cannock Chase Council • An extensive Nature Conservation Area within the site, comprising 50% of the development • New public open spaces and an on-site children’s play area with links through to Chasewater Country Park • The preservation of existing wildlife around the site • The site was sold to Taylor Wimpey for development in 2014 www.richboroughestates.co.uk Richborough Estates Ltd, Waterloo House, 20 Waterloo Street, Birmingham, B2 5TB t +44 (0)121 633 4929 - f +44 (0)121 633 0718 Case Study: Residential Sector Norton Canes Staffordshire Key Benefits • 130 residential units including affordable housing • New nature conservation area • Improvements to the community centre • New off-site grazing land • New open spaces and children’s play area • New footpaths and cycleways T (+44) 0121 633 4929 www.richboroughestates.co.uk. -

66 Brownhills Road, Norton Canes, Ws11 9Se
66 BROWNHILLS ROAD, NORTON CANES, WS11 9SE TO LET BY WAY OF ASSURED SHORTHOLD TENANCY £675.00 p.c.m. THREE BEDROOM DETACHED HOUSE CONVENIENT FOR LOCAL FACILITIES Lounge Landing Cloakroom with WC Three Bedrooms Dining Kitchen Bathroom Gas Central Heating Fully Double Glazed Off Road Parking & Garage To Rear Sorry No Smokers or Sharers THESE PARTICULARS SHOULD BE READ IN CONJUNCTION WITH THE FORMAL NOTICES BELOW 01543 50 54 54 19 Wolverhampton Road, Cannock, Staffordshire. WS11 1DG Facsimile: 01543 466913 E-mail: [email protected] Web: www.bootandson.co.uk 66 Brownhills Road, Norton Canes All measurements given are approximate and for guidance purposes only All photographs have been taken with an extra wide angle lens. GROUND FLOOR LOUNGE - 17ft 8ins x 5ft 11ins to 10ft 1in (5.38 x 1.80 to 3.07) overall into stairwell but excluding door recess, with UPVC double glazed access door and side window, UPVC double glazed window, two radiators, television aerial point, telephone point and ceiling mounted smoke alarm. CLOAKROOM - with low flush WC, pedestal hand basin with tiled splash guard and radiator. DINING KITCHEN - 17ft 7ins x 7ft 0in (5.36 x 2.13) fitted with range of base units, drawers, laminate work surfaces, inset stainless steel sink with mixer taps, wall cupboards, electric built-in oven with gas hob and extractor fan over, ceramic tiled floor, UPVC double glazed window, double glazed sliding patio access door to rear garden and ‘Heatline’ gas fired combination condensing central heating boiler. 66 Brownhills Road, Norton Canes FIRST FLOOR LANDING - with ceiling mounted smoke alarm and giving access to: BEDROOM ONE - 9ft 9ins x 8ft 8ins (2.97 x 2.64) with UPVC double glazed window and radiator. -

Norton Canes Ward Notice Is Hereby Given That: 1
NOTICE OF POLL AND SITUATION OF POLLING STATIONS Cannock Chase District Council Election of a District Councillor for Norton Canes Ward Notice is hereby given that: 1. A poll for the election of a District Councillor for Norton Canes will be held on Thursday 6 May 2021, between the hours of 7:00 am and 10:00 pm. 2. The number of District Councillors to be elected is one. 3. The names, home addresses and descriptions of the Candidates remaining validly nominated for election and the names of all persons signing the Candidates nomination paper are as follows: Names of Signatories Name of Candidate Home Address Description (if any) Proposers(+), Seconders(++) & Assentors ALLEN 77 Church Road, Reform UK Kerry L Bladen (+) Stewart P Bladen (++) Paul Gregory Norton Canes, Cannock, Staffordshire, WS11 9PF HOARE 10 Chapel Street, The Conservative Party Sheila J Harding (+) Alan Harding (++) Mike Norton Canes, Candidate Cannock, Staffordshire, WS11 9NT MAWLE 448 Rawnsley Road, Chase Community Christine J Holmes (+) Albert G Holmes (++) Linda Joy Hednesford, Independents People Staffordshire, WS12 Before Politics 1RB STRETTON MBE 49 Walsall Road, Labour Party John P T L Preece (+) Joshua A A Newbury Zaphne Phyllis Norton Canes, (++) Staffordshire, WS11 9QY TAPPER (Address in Cannock The Green Party Amy Tapper (+) Guy M Thursfield (++) Glen Gary Chase) 4. The situation of Polling Stations and the description of persons entitled to vote thereat are as follows: Station Ranges of electoral register numbers of Situation of Polling Station Number persons entitled to vote thereat Jerome C P School, Hussey Road, Norton Canes, Cannock 64 NC1-1 to NC1-1446 Norton Canes Methodist Church Hall, Poplar Street, Norton 65 NC2-1 to NC2-1147 Canes, Cannock Norton Canes Methodist Church Hall, Poplar Street, Norton 66 NC2-1148 to NC2-2187 Canes, Cannock Norton Canes Community Centre, Brownhills Road, Norton 67 NC3-1 to NC3-1323 Canes Norton Canes Community Centre, Brownhills Road, Norton 68 NC3-1324 to NC3-2531 Canes 5. -

Site Allocations Appendices PAGES 1 to 21
CANNOCK CHASE LOCAL DEVELOPMENT PLAN FRAMEWORK DEVELOPMENT PLAN DOCUMENT SITE ALLOCATIONS – ISSUES AND OPTIONS APPENDICES CABINET DRAFT - FEBRUARY 2007 Appendix 1: Schedule of Suggested Potential Development Sites The sites listed in this schedule have been suggested prior to Friday 15th September 2006 for consideration as potential development sites in this DPD. A plan of each site can be found in Appendix 2 on page 45. The reference numbers e.g. 01.004 relate to individual suggestions, and these may not relate to the whole geographical site number. These sites are also shown on the Issues & Options plan, which accompanies this document. The ‘Status’ column shows whether it is intended to carry each suggestion forward for further consideration toward the Preferred Options stage. Short listed sites at Preferred Options may not correspond with those below. Site Ref. Location Area Suggested Use Suggested by Status No. No. (ha) 1 01.004 Land west of Pye Green Road 65.92 Residential Development Mr. G. Horton Take forward for consideration prior to the Preferred Options stage. 1 01.021 Land west of Pye Green Road 1.357 Residential Development David Wilson Take forward for consideration prior to the (Pye Green Road / Limepit Lane) Homes (West Preferred Options stage. Midlands) 1 01.047 Land west of Pye Green Road 57.38 Residential Development RPS on behalf of Take forward for consideration prior to the St. Modwen Preferred Options stage. Developments Ltd. 1 01.434 Land west of Pye Green Road 3.637 Residential Development Wardell Take forward for consideration prior to the (opposite Thornhill Road / Bond Way) Armstrong Preferred Options stage. -

Pages 23 to 31 of 2019-20)
HednesfordTown Council Gateway to the Chase 3 September 2019 Dear Councillor A Meeting of the Town Council will be held at 7:30 pm on Tuesday 10 September 2019 at Pye Green Community Centre, Bradbury Lane, Hednesford. You are invited to attend for consideration of the matters shown on the agenda Yours sincerely Peter Harrison Town Council Manager/Clerk PUBLIC PARTICIPATION Members of the public are invited to address the Council and ask questions before the meeting begins Additionally, County and District Councillors and local PCSO (if present) AGENDA 1. Apologies 2. Declarations of Interest 3. Minutes - to approve the minutes of the Meeting held on 30 July 2019 (Enclosed pages 23 to 31 of 2019-20) 4. Items for Information and Updates on matters not included in the reports of Principal Speakers or the Town Council Manager/Clerk 5. Chairman's Announcements Peter Harrison JP BA(Hons)Town Council Manager/Clerk Pye Green Community Centre Bradbury Lane Hednesford Staffordshire WS12 4EP [email protected] Tel: 01543 424872 Skype: HTC.clerk 1 6. Reports from Principal Speakers To consider reports from Principal Speakers Community Projects and Events Hednesford Festival Staffordshire Regiment Association (Hednesford Branch) - Freedom March 21 September 2019 Silver Sunday- 6 October 2019 Christmas Event- 6 December 2019 #VE75- 8 to 10 May 2020 Armed Forces Day- 27 June 2020 Homelessness and Vulnerable Adults Fact finding with Cannock Chase District Council and invitation to Housing Options team to meeting Councillors Severe Weather Emergency Protocol Communications and Engagement PR and Media Support-To report that the support provided by the PRwoman has now ceased and to consider arrangements for the future including website refresh and administration. -

3 Bus Time Schedule & Line Route
3 bus time schedule & line map 3 Cannock - Norton Canes - Brownhills View In Website Mode The 3 bus line (Cannock - Norton Canes - Brownhills) has 2 routes. For regular weekdays, their operation hours are: (1) Brownhills: 6:00 AM - 5:45 PM (2) Cannock Town Centre: 6:47 AM - 6:02 PM Use the Moovit App to ƒnd the closest 3 bus station near you and ƒnd out when is the next 3 bus arriving. Direction: Brownhills 3 bus Time Schedule 57 stops Brownhills Route Timetable: VIEW LINE SCHEDULE Sunday Not Operational Monday 6:00 AM - 5:45 PM Bus Station, Cannock Town Centre Tuesday 6:00 AM - 5:45 PM Coniston Way, Cannock 65 Church Street, Cannock Wednesday 6:00 AM - 5:45 PM Hollies Park Road, Cannock Town Centre Thursday 6:00 AM - 5:45 PM Friday 6:00 AM - 5:45 PM Mill Farm Ph, Rumer Hill Saturday 7:05 AM - 5:45 PM Mcarthurglen, Hawks Green Mcarthurglen Designer Outlet, Hawks Green Chaseside Drive, Hawks Green 3 bus Info Direction: Brownhills Oakland Industrial Estate, Hawks Green Stops: 57 Trip Duration: 47 min No 62, Hednesford Line Summary: Bus Station, Cannock Town Centre, Coniston Way, Cannock, Hollies Park Road, Cannock Lower Road, Cannock Town Centre, Mill Farm Ph, Rumer Hill, Mcarthurglen, Hawks Green, Mcarthurglen Designer Outlet, Hawks Hill Street, Hawks Green Green, Chaseside Drive, Hawks Green, Oakland Industrial Estate, Hawks Green, No 62, Hednesford, Cross Keys Ph, Hednesford Hill Street, Hawks Green, Cross Keys Ph, Hednesford, Hill Street, Cannock Charlock Grove, Hawks Green, Tutbury Close, Hawks Green, Rosebay Meadow, Hawks Green, Sidon -

Norton Canes ‘Open Door’ 2018 at Issue 23 St James the Great Parish Church
September Norton Canes ‘Open Door’ 2018 at Issue 23 St James the Great parish Church Charity Bike Ride Challenge Three adventurous ladies from a Norton Canes salon will be braving a gruelling charity bike ride. Tracy Krzyszowski, Amy Large and Samantha Grace, from It’s All About You, on Burntwood Road, are now in Jenny Woodman (centre) training for their 27-mile challenge. with students And you are invited to support their effort which is in aid of St Giles’ Hospice. On September 2 the ladies will set off from St Giles and ride through Walton on Trent, Rosliston, Netherseal, Clifton Campville, Haunton and Harlaston before returning to St Giles in Whittington. They have collection buckets at their salon, at the Yew Tree pub, the village chip shop and the Lido Club in Norton Canes. If you would like to make a donation please call into any of those venues. Pictured with their cycle ride Tracy, aged 56, from St James Road, leaflets are (from left): Tracy, Norton Canes, runs the tanning and Samantha and Amy. sunbed part of the salon. “We have been cycling for a bit of fun to get fit,” she said. “We started at the end of ‘Celestial Light’ by Peter Clark ‘Evening Light’ by David Byrne March and we are getting better. We decided to push ourselves to do something.” Tracy works with Amy, aged 26, from Wolverhampton who has been the salon’s barber for about nine years; and Samantha, aged 40, from Burntwood, who is the BKV results New Zen Garden at the Library see page 8 ‘Final Approach’ by Tony Stamp ‘Winning Smile’ by John Cartlidge nail technician. -

Staffs and Stoke 18.6.21
Telephone Pharmacy Address Postcode Number Asda Pharmacy Asda Superstore Wolstanton Retail Park Wolstanton ST5 0AY 01782 349010 Asda Pharmacy Asda Stores Ventura Park Tamworth B78 3HB 01827 302180 Asda Pharmacy Scotia Road Tunstall Stoke on Trent ST6 6AT 01782 820010 Asda Pharmacy Asda Superstore Queensway Stafford ST16 3TA 01785 782010 Asda Pharmacy The Octagon Centre Orchard Street Burton upon Trent DE14 3TN 01283 523210 Bains Pharmacy 160 - 162 Hednesford Road Heath Hayes Cannock WS12 3DZ 01543 279415 Blurton Pharmacy 8 Ingestre Square Blurton Stoke on Trent ST3 3JT 01782 314408 Blythe Bridge Pharmacy 240 Uttoxeter Road Blythe Bridge Staffordshire ST11 9LY 01782 393127 Bradwell Pharmacy 111 Hanbridge Avenue Bradwell Newcastle U Lyme ST5 8HX 01782 711493 Branston Pharmacy Main Street Branston Burton upon Trent DE14 3EY 01283 569624 Chasetown Pharmacy 23 High Street Chasetown Staffordshire WS7 8XE 01543 682921 Cornwell's Chemists Holmcroft Road Stafford Staffordshire ST16 1JG 01785 250151 Cornwell's Chemists 51 Bodmin Avenue Weeping Cross Stafford ST17 0EF 01785 661765 Cornwell's Chemists Weston Road Stafford Staffordshire ST18 0BF 01785 247361 Cornwell's Chemists 11 High Street Newcastle under Lyme Staffordshire ST5 1RB 01782 663921 Cornwell's Chemists 126 Wardles Lane Great Wyrley Walsall WS6 6DZ 01922 414060 Cornwell's Chemists 235 Cannock Road Chadsmoor Cannock WS11 2DD 01543 509434 Coven Pharmacy 25 Brewood Road Coven Wolverhampton WV9 5BX 01902 790074 Day Night Pharmacy Unit 4 Swan Island Shopping PrecinctChase Road WS7 0DW 01543 -

Walkmill Place Cannock
Walkmill Place Cannock lindenhomes.co.uk Walkmill Place Cannock A collection of 2, 3 & 4 bedroom homes in a pretty market town Walkmill Place is located in Cannock, a charming Staffordshire Walkmill Place is very well located for easy access to major town ideal for all kinds of buyer. Close to Cannock Chase, an area road networks. Wolverhampton, Stafford and Walsall are all of Outstanding Natural Beauty, this well-appointed development within a 12 mile radius, with Birmingham only 20 miles away. of 2, 3 and 4 bedroom homes is in an enviable location. You will find The M5, M6, M6 Toll and M54 motorways are all within easy a variety of shops, pubs, restaurants and cafes nearby to cater for reach for onward journeys. all your needs. Travelling by rail is easy too. Cannock train station is just Parents of growing families will find schools close by for all ages a 6 minute drive from Walkmill Place, and offers services which are highly rated by Ofsted. to Birmingham, London Euston and other UK locations. Walkmill Place Walkmill Lane, Cannock WS11 0FJ | 01543 330 189 lindenhomes.co.uk/walkmillplace D A R O AW 1 R N L N S IME O L PIT LANE I E Y T A R E O T A N S D A L D A B W EL O T O R R O R A R N D E Hednesford A P E S R A D K A G O 3 R C EWORT H 4 E L O T Y IT C P L 60 A4 S T EV CANNOCK E EN E S B R R 5 O T A 0 D S 1 B 2 4 N 1 5 H 4 2 O J Y A W S E L HEATH HAYES L O 3 4 B N A G T F 190 CANNOCK R S O A5 OAD R Cannock D R O H A E D 5 D N E S 4 0 A F 5 3 6 A O 4 D M R A A I D O N R E R D N O R O O S O A W R W T D T A O N Y N R 6 L U A A B 5 N 7 E 8 9 TOLL 6 A M M 5 6 NORTON CANES TO LL A 3 4 D 10 A O 0 R 6 N TOLL 4 M6 O A H 11 ER AM T A 12 WO LV P 5 CHESLYN HAY GREAT WYRLEY Around the local neighbourhood 1 Cannock Chase 5 Cannock Train Station 9 Planet Ice Cannock 2 Chase Leisure Centre 6 Bridgtown Primary School 10 St Mark’s Church 3 Cannock Shopping Centre 7 Orbital Retail Park 11 Chasewater Country Park Mill Green & Hawks Green Valley Sainsbury’s Supermarket Cheslyn Hay Academy 4 Nature Reserve 8 12 Distances shown are by road (Source: Google).