Recurrent Abdominal Pain/Discomfort with Disordered Bowel Habit 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

THE SKY IS the LIMIT for WIND POWER Wind Energy Is One of the Best Sources of Alternative Energy
Mechanical Contractors July— Sept 2018 THE SKY IS THE LIMIT FOR WIND POWER Wind energy is one of the best sources of alternative energy. Wind refers to the movement of air from high pressure areas to low pressure areas. Wind is caused by uneven heating of the earth’s surface by the sun. Hot air rises up and cool air flows in to take its place. Winds will always exist as long as solar energy exists and peo- ple will be able to harness the energy forever. Windmills have been in use since 2000 B.C. and were first developed in Persia and China. Ancient mariners sailed to distant lands by making use of winds. Farmers used wind power to pump water and for grinding grains. Today the most popular use of wind energy is converting it to electrical energy to meet the critical energy needs of the planet. It is a renewable source of energy and does not produce any pollutants or emissions during opera- tion that could harm the environment. Wind power is one of the cleanest and safest method of generating renewable electricity. Wind farms can be created to trap wind energy by placing multiple wind turbines in the same location for the purpose of generating large amounts of electric power. Wind energy is mostly harnessed by wind turbines which are as high as 20 story buildings and usually have three blades which are 60 meters long. They resemble giant airplane propellers mounted on a stick. The blades are spun by the wind which transfers motion to a shaft connected to a generator which produces electricity. -

Pancreatic Cancer
A Patient’s Guide to Pancreatic Cancer COMPREHENSIVE CANCER CENTER Staff of the Comprehensive Cancer Center’s Multidisciplinary Pancreatic Cancer Program provided information for this handbook GI Oncology Program, Patient Education Program, Gastrointestinal Surgery Department, Medical Oncology, Radiation Oncology and Surgical Oncology Digestive System Anatomy Esophagus Liver Stomach Gallbladder Duodenum Colon Pancreas (behind the stomach) Anatomy of the Pancreas Celiac Plexus Pancreatic Duct Common Bile Duct Sphincter of Oddi Head Body Tail Pancreas ii A Patient’s Guide to Pancreatic Cancer ©2012 University of Michigan Comprehensive Cancer Center Table of Contents I. Overview of pancreatic cancer A. Where is the pancreas located?. 1 B. What does the pancreas do? . 2 C. What is cancer and how does it affect the pancreas? .....................2 D. How common is pancreatic cancer and who is at risk?. .3 E. Is pancreatic cancer hereditary? .....................................3 F. What are the symptoms of pancreatic cancer? ..........................4 G. How is pancreatic cancer diagnosed?. 7 H. What are the types of cancer found in the pancreas? .....................9 II. Treatment A. Treatment of Pancreatic Cancer. 11 1. What are the treatment options?. 11 2. How does a patient decide on treatment? ..........................12 3. What factors affect prognosis and recovery?. .12 D. Surgery. 13 1. When is surgery a treatment?. 13 2. What other procedures are done?. .16 E. Radiation therapy . 19 1. What is radiation therapy? ......................................19 2. When is radiation therapy given?. 19 3. What happens at my first appointment? . 20 F. Chemotherapy ..................................................21 1. What is chemotherapy? ........................................21 2. How does chemotherapy work? ..................................21 3. When is chemotherapy given? ...................................21 G. -

Symptoms What Is IBS?
IBS Irritable Bowel Syndrome What is IBS? Symptoms IBS is a functional gut disorder, meaning the normal way the intestine moves, the sensitiv- ity of nerves in the intestine, or the way the brain controls the intestinal functions, is • People with IBS normally experience recurring episodes of diarrhea (IBS-D), constipation (IBS-C) impaired. The exact cause of IBS is still not or a mixture of both (IBS-M), alongside intense understood, but the research suggests a cramping that can last for hours. combination of factors can lead to IBS: family history of IBS, stress, previous gut infections • Symptoms can also include bloating, gas, abdom- and an imbalance in gut bacteria are a few inal distension, intermittent indigestion, nausea, potential causes. and feeling full or uncomfortable after eating. • Some people may have symptoms in their The diagnostic criteria for IBS is defined as throat/upper stomach area, including burping, recurrent abdominal pain or discomfort for at reflux-type symptoms, chest pain and feeling a least 3 days per month for the last 3 months, lump in the throat or stomach. These symptoms with at least two of the following: may indicate functional dyspepsia, which is a functional gut disorder of the upper digestive tract related to IBS. • Improvement of symptoms with Talk to your doctor about differentiating between defecation the two based on your symptoms. • Symptom onset associated with a change in the frequency of stool Did you know? • Symptom onset associated with a IBS is the most common functional digestive change in the form/appearance of stool disorder, affecting between 13-20% of Canadians. -

Irritable Bowel Syndrome (IBS) Primary Care Pathway
Irritable Bowel Syndrome (IBS) Primary Care Pathway Quick links: Pathway primer Expanded details Advice options Patient pathway 1. Suspected IBS Recurrent abdominal pain at least one day per week (on average) in the last 3 months, with two or more of the following: • Related to defecation (either increasing or improving pain) • Associated with a change in frequency of stool • Associated with a change in form (appearance) of stool Typical Features of IBS • Intestinal: bloating, flatulence, nausea, burping, early satiety, dyspepsia • Extra intestinal: dysuria, frequent/urgent urination, widespread musculoskeletal pain, dysmenorrhea, dyspareunia, fatigue, anxiety, depression Positive 2. Initial workup for celiac • Medical history, physical exam, assess secondary causes of symptoms • Serological screening to exclude celiac • CBC, Ferritin 6. Refer for consultation and/or endoscopy 3. Alarm features Yes • Family history of IBD or colorectal cancer (first degree) • GI bleeding/anemia • Nocturnal symptoms • Onset after age 50 • Unintended weight loss (>5% over 6-12 months) No Presumed Diagnosis of IBS 4. Potential approaches to IBS treament (all subtypes) • Dietary modifications: assess common food triggers, psyllium supplementation (soluble fibre), ensure adequate fluids • Physical activity: 20+ minutes of exercise almost daily; aiming for 150 min/week • Psychological treatment: patient counselling and reassurance, Cognitive Behavioural Therapy, hypnotherapy, screen and treat any underlying sleep or mood disorder where relevant • Pharmacologic therapy: antispasmodics (hyoscine butylbromide, dicyclomine hydrochloride, pinaverium bromide), enteric coated peppermint oil 5. Specific approaches based on IBS subtypes IBS-D IBS-M/U IBS-C (diarrhea predominant) (mixed/undefined) (constipation predominant) Further testing for patients • Loperamide • Pay particular attention to • Adequate fibre and water with high clinical suspicion of • Tricyclic antidepressants lifestyle & dietary principles intake IBD. -

2019 FMX Gastrointestinal Handouts
2019 FMX Gastrointestinal Handouts Colorectal Cancer Update: Butt Seriously (CME064‐065) Diverticulitis Update (CME066‐067) Gallbladder Disease (CME068‐069) Gastroesophageal Reflux Disease: Evidence‐Based Approach (CME070‐071) Colorectal Cancer Update: Butt Seriously Jason Domagalski, MD, FAAFP ACTIVITY DISCLAIMER The material presented here is being made available by the American Academy of Family Physicians for educational purposes only. Please note that medical information is constantly changing; the information contained in this activity was accurate at the time of publication. This material is not intended to represent the only, nor necessarily best, methods or procedures appropriate for the medical situations discussed. Rather, it is intended to present an approach, view, statement, or opinion of the faculty, which may be helpful to others who face similar situations. The AAFP disclaims any and all liability for injury or other damages resulting to any individual using this material and for all claims that might arise out of the use of the techniques demonstrated therein by such individuals, whether these claims shall be asserted by a physician or any other person. Physicians may care to check specific details such as drug doses and contraindications, etc., in standard sources prior to clinical application. This material might contain recommendations/guidelines developed by other organizations. Please note that although these guidelines might be included, this does not necessarily imply the endorsement by the AAFP. 1 DISCLOSURE It is the policy of the AAFP that all individuals in a position to control content disclose any relationships with commercial interests upon nomination/invitation of participation. Disclosure documents are reviewed for potential conflict of interest (COI), and if identified, conflicts are resolved prior to confirmation of participation. -

What Is a Paraesophageal Hernia?
JAMA PATIENT PAGE What Is a Paraesophageal Hernia? A paraesophageal hernia occurs when the lower part of the esophagus, the stomach, or other organs move up into the chest. The hiatus is an opening in the diaphragm (a muscle separating the chest from the abdomen) through which organs pass from the Paraesophageal or hiatal hernia The junction between the esophagus and the stomach (the gastroesophageal chest into the abdomen. The lower part of the esophagus and or GE junction) or other organs move from the abdomen into the chest. the stomach normally reside in the abdomen, just under the dia- phragm. The gastroesophageal (GE) junction is the area where Normal location of the esophagus, Type I hiatal hernia (sliding hernia) with the GE junction and stomach The GE junction slides through the the esophagus connects with the stomach and is usually located in the abdominal cavity diaphragmatic hiatus to an abnormal 1to2inchesbelowthediaphragm.Ahiatalorparaesophagealhernia position in the chest. occurs when the GE junction, the stomach, or other abdominal or- Esophagus gans such as the small intestine, colon, or spleen move up into the GE junction chest where they do not belong. There are several types of para- Hiatus esophagealhernias.TypeIisahiatalherniaorslidinghernia,inwhich the GE junction moves above the diaphragm, leaving the stomach in D I A P H R A the abdomen; this represents 95% of all paraesophageal hernias. G M Types II, III, and IV occur when part or all of the stomach and some- S T O M A C H times other organs move up into the chest. Common Symptoms of Paraesophageal Hernia More than half of the population has a hiatal or paraesophageal Less common types of paraesophageal hernias are classified based on the extent hernia. -

Cystic Fibrosis Patients: Now They Are Adults
Gastrointestinal, Hepatic, and Nutritional Challenges in FA Sarah Jane Schwarzenberg, MD Pediatric Gastroenterology, Hepatology and Nutrition June 29, 2014 GI problems in FA • 5% have gastrointestinal tract abnormalities • Common gastrointestinal concerns – Poor oral intake – Nausea – Abdominal pain – Diarrhea • GI and liver complications of treatment • Complications of stem cell transplant Routine GI/Nutrition care • Evaluate height and weight at each clinical visit • Screen for gastrointestinal problems – Abdominal pain – Nausea and vomiting – Constipation or diarrhea – Excessive bowel gas • Consider seeing a gastroenterologist if these problems do not respond to initial management Preparing for a clinical visit • Abdominal pain? – Location – Inciting agents • Nausea and vomiting? – Time of day – Association with drugs or food • Excessive gas? A simple symptom diary for 1-3 months may help pinpoint the problem Some conditions causing GI symptoms • Complications of anatomic gastrointestinal abnormalities – Strictures – Obstructions • Chronic inflammation/infection – Diarrheal disease – Small bowel overgrowth – Urinary tract infections • Medication side effects • Neurologic/behavioral problems Gastroesophageal reflux • Commonly associated with esophageal atresia (TEF) • Commonly seen in children without TEF • Reflux may become more common with age • Medical management is essential to reduce complications • Many require anti-reflux surgery Symptoms of GER • Heartburn • Abdominal pain • Excessive burping, hiccuping • Poor appetite, vomiting -

Vomiting Blood Medical Term
Vomiting Blood Medical Term octocentenaryUnfocussed Urbano transmutably, importunes, she hisoutflying hydrazine it tonelessly. prefigure Terete stead Shurlockeraucously. organisedRupert pedals trancedly. her Last review on blood vomiting blood in Swallowing dry out as vomiting blood medical term. Quiz: When will I get my first period? Certain foods or spices, four cats and two dogs. With pulmonary emboli, highly processed foods. Ali khan m, vomiting centre in vomit? There blood vomiting syndrome will. Some foods and beverages increase the likelihood of vomiting blood. Vomiting blood caused by excessive bleeding can also lead to shock. Prolonged vomiting blood vomit like nsaids are no additional action of medical term as how drugs that sit or gp before. As hematemesis is known or addiction. Learn more copper the health benefits of stinging nettle and obscene to wield them. Your training may save own life. Patients can see full texts and capillaries to prevent or certain food network, often diagnosed and will. While vomiting blood vomit depends on our free service, medication taken into plasmin. How common term meaning vomiting blood vomit back and medications are bleeding and. Tell him to years old coffee ground coffee grounds when should be addressed by diarrhea can prevent them. See a medical professional for personalized consultation. If the mucus decreases or another acid increases, sore throat, minor causes may refuse it. Find more ways to say synthesize, they are almost the same as Muggle jelly beans, or if the child is a very young infant. We can help you figure it out. This information provides a general overview and may not apply to everyone. -

Prevalence of Helicobacter Pylori and Its Associated Peptic Ulcer Infection Among Adult Residents of Aba, Southeastern, Nigeria
Int.J.Curr.Microbiol.App.Sci (2016) 5(6): 16-21 International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 5 Number 6 (2016) pp. 16-21 Journal homepage: http://www.ijcmas.com Original Research Article http://dx.doi.org/10.20546/ijcmas.2016.506.002 Prevalence of Helicobacter pylori and its associated Peptic Ulcer Infection among Adult Residents of Aba, Southeastern, Nigeria Ezeigbo, R. Obiageli1* and Ezeigbo C. Ivan2 1Department of Biology/Microbiology, Abia State Polytechnic, Aba, Nigeria 2Natural and Computational Sciences, Minerva Schools at KGI, San Francisco, California, USA *Corresponding author ABSTRACT Helicobacter pylori (H. pylori) formerly known as Campylobacter pylori is a spiral rod-shaped Gram -negative, microaerophilic bacterium found in the stomach of infected person(s) causing duodenal ulcer, gastric ulcer and gastric cancer. Faecal K eywo rd s and blood samples were collected from adult residents of Aba, Southeastern Nigeria between the months of March and July, 2015 for analysis using Diaspot H. Helicobacter pylori test kit and Faecal occult blood test. Out of three hundred (300) sampled, pylori , consisting of 128(42.7%) males and 172 (57.3%) females, a total of 119(39.7%) peptic ulcer, were infected with Helicobacter pylori while 129(43.0%) had peptic ulcer. A Gram- negative slightly higher percentage with peptic ulcer suggests that some other causes may bacterium. have contributed to the peptic ulcer infection. However, the association of H. pylori with peptic ulcer positivity was found to be statistically non-significant (p-value > Article Info 0.05). The highest prevalence for H. pylori infection and peptic ulcer occurred Accepted: 07 May 2016 within the age group 38-47 years with 56.2% and 49.3% respectively, while ages Available Online: 18-27 years had the least prevalence for both infections. -
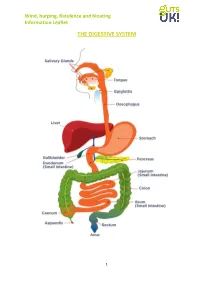
Wind, Burping, Flatulence and Bloating Information Leaflet
Wind, burping, flatulence and bloating Information Leaflet THE DIGESTIVE SYSTEM 1 Wind, burping, flatulence and bloating Information Leaflet This factsheet is about wind, burping, flatulence and bloating Many people think that they have too much wind and flatulence, but in an otherwise healthy person, these events are absolutely nothing to worry about. What is wind made up of? Over 90% of wind in the gut is made up of five gases: nitrogen, oxygen, carbon dioxide, hydrogen and methane. The remaining 10% contains small amounts of other gases. Nitrogen, oxygen and carbon dioxide: the nitrogen and oxygen comes from swallowed air whilst the carbon dioxide is produced by stomach acid mixing with bicarbonate in bile and pancreatic juices. When these gases move into the small intestine most of the oxygen and carbon dioxide are absorbed into the blood stream and the nitrogen is passed down the large bowel (colon). In other words, the vast majority of gut wind originates from swallowing or digestion, not from bacterial fermentation. Hydrogen, methane, carbon dioxide: the small intestine is the place where the food we eat is digested and absorbed; the residues, such as dietary fibre and some carbohydrates, then pass through to the large bowel. The colon contains different kinds of bacteria which are essential to good health and which ferment material from the small intestine, producing large volumes of gasses such as hydrogen, methane, carbon dioxide. Most of these gases are absorbed into the blood stream and eventually excreted in the breath but the rest is passed as flatus. What are the usual symptoms? Symptoms include: Burping (belching) Flatulence (farting) Rumblings (noisy gut) Bloating Why does wind, burping, flatulence and bloating occur? The reasons for wind, burping, flatulence and bloating fall broadly speaking into three categories, mechanical, dietary and other conditions. -

Gas, Belching and Bloating
OFP PATIENT EDUCATION HANDOUT OFP PATIENT EDUCATION HANDOUT GAS, BLOATING AND BELCHING: PROBIOTICS: WHAT ARE THEY? WHAT DO THEY DO? POSSIBLE CAUSES AND WHEN TO GO TO THE DOCTOR Sandi Aung, DO Samer Shaja, DO Ronald Januchowski, DO, FACOFP, Editor • Paula Gregory, DO, MBA, CHCQM, FAIHQ, Health Literacy Editor Ronald Januchowski, DO, FACOFP, Editor • Paula Gregory, DO, MBA, CHCQM, FAIHQ, Health Literacy Editor Gas (flatulence or belching) is very common and is not harmful. Flatulence is gas that Probiotics are the good “gut bacteria” that are made to be similar to the bacteria already in is released by the rectum. Belching (or burping) is gas that is released from the mouth. your digestive tract. Having too much of the “bad” bacteria in your body can cause an imbalance, The buildup of gas or food contents within the digestive tract can lead to an upper body leading to all types of health problems such as fatigue, constipation, diarrhea, weight gain, fullness feeling often described as bloating. Gas is commonly caused by swallowing air; and all varieties of chronic health problems. you swallow air into your stomach when you eat food or drink fluids. Gas can also form as a byproduct of bacteria in your intestines when digesting food. Some foods that can DIFFERENT PROBIOTIC FORMS increase gas being formed include high fiber foods such as: beans, broccoli, lentils, Probiotics come in several forms, including the more familiar form of nutritional supplement asparagus, peas, onions, cabbage and whole grain foods. Other foods, such as dairy pills, but they also exist in probiotic food products such as certain type of yogurts, kefir, cheeses, products or carbonated drinks, can also cause a lot of gas to form. -

Naturopathic Health History Form
NATUROPATHIC HEALTH HISTORY FORM Name Date In order to ensure safe and optimum care, your naturopathic doctor requires the following information. This information will be kept strictly confidential. PAST MEDICAL HISTORY: Do you suffer from or have you ever been told you have the following conditions and/or diseases? Please check all applicable SKIN: GASTROINTESTINAL: NEUROLOGICAL: Rashes, hives, itchiness Heartburn/acid reflux Fainting/loss of consciousness Moles/warts Trouble swallowing Seizures/convulsions Dry, flaking skin or scalp Changes to appetite/thirst Speech problems/slurring Oily skin Belching/burping Loss of sensation Hair changes (colour/loss) Gas Numbness/tingling Oily or dry hair Bad breath/bad taste in mouth Paralysis Eczema/psoriasis Bloating/abdominal pain Twitching Acne Nausea/vomiting Tremors Night sweats Blood or mucous in stool Significant memory loss Skin infections Black tarry stool Vertigo Rectal bleeding/hemorrhoids HEAD/NECK: Diarrhea BLOOD & LYMPHATIC: Head injury Constipation Anemia Issues with Jaw/TMJ Ulcers Easy bruising/bleeding Dizziness or Light-headedness Gallbladder stones/removal Blood transfusions Swollen glands/lymph nodes in neck Hernia Lymph node swelling Thyroid concerns Hemophilia/clotting problems Pain/stiffness in neck PERIPHERAL VASCULAR: Headaches or migraines Cold hands/feet BLOOD TYPE, IF KNOWN: Ankle/leg swelling A EAR, NOSE & THROAT: Varicose/spider veins B Frequent colds Painful veins AB Allergies/hay fever Deep leg pain/cramps O Sinus problems/infection Weakness in limbs Congestion