A Management Plan for Ballyvergan Marsh, Youghal, Co. Cork
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Baldwins of Lisnagat : Work in Progress
The Baldwins of Lisnagat : Work in Progress Alexandra Buhagiar 2014 CONTENTS Tables and Pictures Preamble INTRODUCTION Presentation of material Notes on material Abbreviations Terms used Useful sources of information CHAPTER 1 Brief historical introduction: 1600s to mid-1850s ‘The Protestant Ascendancy’ The early Baldwin estates: Curravordy (Mount Pleasant) Lisnagat Clohina Lissarda CHAPTER 2 Generation 5 (i.e. most recent) Mary Milner Baldwin (married name McCreight) Birth, marriage Children Brief background to the McCreight family William McCreight Birth, marriage, death Education Residence Civic involvement CHAPTER 3 Generation 1 (i.e. most distant) Banfield family Brief background to the Banfields Immediate ancestors of Francis Banfield (Gen 1) Francis Banfield (Gen 1) Birth, marriage, residence etc His Will Children (see also Gen 2) The father of Francis Banfield Property Early Milners CHAPTER 4 Generation 2 William Milner His wife, Sarah Banfield Their children, Mary, Elizabeth and Sarah (Gen. 3. See also Chapter 5) CHAPTER 5 Generation 3 William Baldwin Birth, marriage, residence etc Children: Elizabeth, Sarah, Corliss, Henry and James (Gen. 4. See also Chapter 6) Property His wife, Mary Milner Her sisters : Elizabeth Milner (married to James Barry) Sarah Milner CHAPTER 6 Generation 4 The children of William Baldwin and Mary Milner: Elizabeth Baldwin (married firstly Dr. Henry James Wilson and then Edward Herrick) Sarah Baldwin (married name: McCarthy) Corliss William Baldwin Confusion over correct spouse Property Other Corliss Baldwins in County Cork Henry Baldwin James Baldwin Birth, marriage, residence etc. Property His wife, Frances Baldwin CHAPTER 7 Compilation of tree CHAPTER 8 Confusion of William Baldwin's family with that of 'John Baldwin, Mayor of Cork' Corliss Baldwin (Gen 4) Elizabeth Baldwin (Gen 4) CHAPTER 9 The relationship between ‘my’ William Baldwin and the well documented ‘John Baldwin, Mayor of Cork’ family CHAPTER 10 Possible link to another Baldwin family APPENDIX 1. -

The Design and Construction of New Mizen Head Footbridge
The Design and Construction of New Mizen Head Footbridge Murt Coleman, BE CEng FIEI, Chartered Engineer Managing Director, Carillion Irishenco Ltd. Enda Collery, BA BAI CEng MIEI, Chartered Engineer Contracts Manager, Carillion Irishenco Ltd. Eoghan Lehane, BE Eur Ing CEng MIEI MCIWEM, Chartered Engineer Civil Engineering and Property Manager, Commissioners of Irish Lights Brendan Minihane, BE Eur Ing CEng MIEI, Chartered Engineer Project Resident Engineer, Cork County Council Ross O’Donovan, Dip Eng BEng CEng MIEI, Chartered Engineer Senior Resident Engineer, RPS Consulting Engineers Ltd. Noel O’Keeffe, BE Eur Ing CEng FIEI MICE, Chartered Engineer County Engineer, Cork County Council Kevin Power, BE CEng FIEI MICE MCIWEM, Chartered Engineer Director, RPS Consulting Engineers Ltd. Kieran Ruane*, BE MSc(Eng) CEng MIStructE MIEI MICE, Chartered Engineer Technical Director, RPS Consulting Engineers Ltd. Paper first presented to a joint meeting of Engineers Ireland, Institution of Structural Engineers and The Irish Concrete Society in Cork on 08.03.2011. *Corresponding author: Tel +353 (0) 21 4665900, [email protected]. Design and Construction of New Mizen Head Footbridge Paper first presented to Engineers Ireland, IStructE and ICS in Cork on 08.03.2011 The Design and Construction of New Mizen Head Footbridge Overview On the 18th of October 1907, sanction was given for the erection of a reinforced concrete Mizen Head Footbridge in County Cork is a bridge to give access to the island. reinforced concrete through-arch structure Construction of the bridge commenced in 1908 spanning 50m. The original structure was and was completed in 1909. The Contractor completed in 1909. -
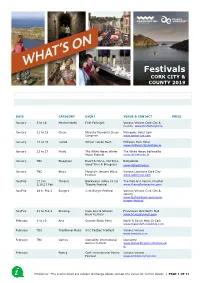
Whats on CORK
Festivals CORK CITY & COUNTY 2019 DATE CATEGORY EVENT VENUE & CONTACT PRICE January 5 to 18 Mental Health First Fortnight Various Venues Cork City & County www.firstfortnight.ie January 11 to 13 Chess Mulcahy Memorial Chess Metropole Hotel Cork Congress www.corkchess.com January 12 to 13 Tattoo Winter Tattoo Bash Midleton Park Hotel www.midletontattooshow.ie January 23 to 27 Music The White Horse Winter The White Horse Ballincollig Music Festival www.whitehorse.ie January TBC Bluegrass Heart & Home, Old Time, Ballydehob Good Time & Bluegrass www.ballydehob.ie January TBC Blues Murphy’s January Blues Various Locations Cork City Festival www.soberlane.com Jan/Feb 27 Jan Theatre Blackwater Valley Fit Up The Mall Arts Centre Youghal 3,10,17 Feb Theatre Festival www.themallartscentre.com Jan/Feb 28 to Feb 3 Burgers Cork Burger Festival Various Venues Cork City & County www.festivalscork.com/cork- burger-festival Jan/Feb 31 to Feb 2 Brewing Cask Ales & Strange Franciscan Well North Mall Brew Festival www.franciscanwell.com February 8 to 10 Arts Quarter Block Party North & South Main St Cork www.makeshiftensemble.com February TBC Traditional Music UCC TadSoc Tradfest Various Venues www.tradsoc.com February TBC Games Clonakilty International Clonakilty Games Festival www.clonakiltygamesfestival.co m February Poetry Cork International Poetry Various Venues Festival www.corkpoetryfest.net Disclaimer: The events listed are subject to change please contact the venue for further details | PAGE 1 OF 11 DATE CATEGORY EVENT VENUE & CONTACT PRICE Feb/Mar -

Development Lands, Chickley's Road, Youghal, Co. Cork
FOR SALE Development Lands, Chickley’s Road, Youghal, Co. Cork. Youghal Town Centre Youghal RFC Pobalscoil na Tríonóide Development Opportunity 2.75 ha (6.8 acres) approx. Property Highlights Contact Séamus Costello • Superb Development Opportunity Email: [email protected] Tel: +353 (0)21 427 5454 • Youghal is a popular east Cork town, occupying a spectacular cushmanwakefield.com seaside setting and benefits from a desirable range of services and amenities Joint Agent • Attractive and established residential suburban location, only 1.5 km from the town centre, with all amenities close by Fiona Hennessy Sherry FitzGerald Hennessy • Parcel of greenfield lands with Phase 1 of Na Prapóga developed Email: [email protected] Tel: +353 (0)24 92595 • The site is located close to the N25 interchange offering excellent accessibility to all arterial routes sherryfitz.ie • Zoned residential and past planning permission that accommodated 74 residential units The Location Price Youghal is an East Cork County town with a population On application. of 8,000 persons approx. It is situated on the N25, 50km east of Cork city. Youghal acts as a service centre for the town and its Viewings surrounding hinterland and is well serviced by retail, View by appointment with the joint agents. local services, schools, sporting and leisure facilities. The subject property is located 1.4km west of Youghal town centre. Description Development opportunity comprising 2.75 ha (6.8 acres) approx. of greenfield undeveloped lands (overall folio being sold is 3.25 ha (8.03 acres). The first phase of No Prapóga that has been completed, provides an attractive entrance to the undeveloped lands. -

Sea Environmental Report the Three
SEA ENVIRONMENTAL REPORT FOR THE THREE PENINSULAS WEST CORK AND KERRY DRAFT VISITOR EXPERIENCE DEVELOPMENT PLAN for: Fáilte Ireland 88-95 Amiens Street Dublin 1 by: CAAS Ltd. 1st Floor 24-26 Ormond Quay Upper Dublin 7 AUGUST 2020 SEA Environmental Report for The Three Peninsulas West Cork and Kerry Draft Visitor Experience Development Plan Table of Contents List of Abbreviations ................................................................................................v Glossary ..................................................................................................................vii SEA Introduction and Background ..................................................... 1 1.1 Introduction and Terms of Reference ........................................................................... 1 1.2 SEA Definition ............................................................................................................ 1 1.3 SEA Directive and its transposition into Irish Law .......................................................... 1 1.4 Implications for the Plan ............................................................................................. 1 The Draft Plan .................................................................................... 3 2.1 Overview ................................................................................................................... 3 2.2 Relationship with other relevant Plans and Programmes ................................................ 4 SEA Methodology .............................................................................. -

Clonakilty Lodge in Co. Cork
Clonakilty Lodge in Co. SLIGO Cork OFFALY Clonakilty Lodge Accommodation Centre is located in Clonakilty in County Cork which is in the south-west of Ireland. The centre houses families. COUNTY CORK Centre Manager: Michael Plichta Public Health Nurse: Anne Marie Hegarty Community Welfare Officer: Mary O’Mahony Jesuit Refugee Service Ireland LOCAL SERVICES PUBLIC SERVICES Social Welfare Citizen’s Information Service Unit 2, Supervalu Shopping Centre, 80 South Mall, Cork City Faxbridge, Clonakilty, Co. Cork Email: [email protected] Phone: 0238821210 Free legal advice available first and third Clonakilty Garda Station Wednesday of every month 18.30 – 19.30 McCurtain Hill, Scartagh, Clonakilty, Co. Cork Phone: 023 882 1570 VOLUNTEERING AND EDUCATION Cork Volunteer Centre Clonakilty College of Further Education 13 North Main Street, Cork City Western Road, Clonakilty, Co. Cork Phone: 0214251572 Phone: 023-8833877 Cork City Adult Guidance Service Email: [email protected] 22 South Mall, Cork City Clonakilty Library Phone: 0214907149 Kent St, Maulnaskehy, Clonakilty, Co. Cork Welcome English Language Centre Phone: 023 883 4275 Free English lessons in Cork City. Phone: 0872281584 / 0214316537 SUPPORT GROUPS Nasc, Irish Immigrant Support LINC (LBGT Women) Centre 11A White Street, Cork City Website: www.nascireland.org www.linc.ie Phone: 0214503462 Phone: 0214808600 Email: [email protected] Email: [email protected] UP Cork LGBT Service (Ages 15-24) The Cork Migrant Centre 4 South Terrace, Cork 14 George’s Quay, Cork City Phone: 0214399862 Phone: 0868246087 Email: [email protected] Email: [email protected] Cork Gay Project (Men) Clonakilty Friends of Asylum Seekers 4 South Terrace, Cork City https://www.facebook.com/ClonFOAS/ Website: www.corkgayproject.com National LGBT Support Line Phone: 0214300430 1890 929 539 Email: [email protected] CHILD AND FAMILY Dunmanway Family Resource Centre For information on schools in the area Kilbarry Road, Dunmanway, Co. -

Strategic Development Opportunity
STRATEGIC DEVELOPMENT OPPORTUNITY Fermoy Co. Cork FOR SALE BY PRIVATE TREATY (AVAILABLE IN ONE OR MORE LOTS) DEVELOPMENT LAND FOR SALE SALE HIGHLIGHTS > Total site area extends to approximately 3.4 ha (8.4 acres). > Zoned Town Centre Mixed Use in the Fermoy Town Centre Development Plan. > Excellent location in the heart of Fermoy Town Centre. > Conveniently located approximately 35kms north east of Cork City Centre. > Location provides ease of access to the M8 and N72. > For sale in one or more lots. LOCATION MAP LOT 1 LOT 2 FERMOY, CO. CORK THE OPPORTUNITY DISTANCE FROM PROPERTY Selling agent Savills is delighted to offer for M8 3km sale this development opportunity situated in the heart of Fermoy town centre within N72 Adjacent walking distance of all local amenities. The Jack Lynch Tunnel & M8 29km property in its entirety extends to 3.4 ha (8.4 acres), is zoned for Town Centre development Cork City Centre 35km and is available in one or more lots. The site is Little Island 32km well located just off Main Street Fermoy with ease of access to the M8, the main Cork to Kent railway station 24km Dublin route. The opportunity now exists to Cork Airport 30km acquire a substantial development site, in one or more lots, with value-add potential in the Pairc Ui Chaoimh 25km heart of Fermoy town centre. Mallow 30km Doneraile Wildlife Park 29km LOCATION Mitchelstown 20km The subject property is located approximately 35km north east of Cork City Centre and approximately 30km east of Mallow and approximately 20km south of Mitchelstown. -

William, James, Hanora, Michael, John, Daniel
The Kellys The Kellys he Kelly family has Irish roots. An examination of Irish records shows that as recently as 1900, TKelly was the second most common name in Ireland (Murphy was fi rst) and there were Kellys located in many areas of Ireland. According to the “Irish Family Names Directory,” Kelly was predominantly found in the counties of Derry, Galway, Leix and Meath, with Galway being the largest concentration. Signifi cant numbers can also be identifi ed with Counties Donegal and Roscommon. These are all areas that are in the midlands to northern regions of the country towards the West. There are also some Kellys who hailed from West Cork where the famous IRA revolutionary Michael Collins called “home.” All of these areas were Gaelic-speaking well into the 1800s. Prior research about the Kellys was unclear about the exact location of their home in Ireland. It remains unspecifi ed but we have made progress (see below). Various individual recollections formed most of the evidence, including my aunt & cousin Bessie Kelly Beirne (1893-1986). Some of these indicate that our Kelly ancestors declared that they were from Clonakilty, County Cork, Ireland. The Immigration and Naturalization Service and several census year records indicate the year of immigration but not the specifi c birthplace. Some local cemeteries and headstones as well as some newspapers record the Kelly origins as Ireland, County Cork and/or Ballymacarder (sic). It should be noted that none of our immigrant ancestors could read or write and may have responded to census and INS questions with the last location that they recall in Ireland. -

Ballysallagh Industrial Estate, Charleville, Co. Cork
For Sale I Industrial Premises N Ballysallagh Industrial Estate D Charleville U Co. Cork S T R I A L • Detached modern purpose built industrial premises comprising c. 2,213 Sq. M. (23,818 sq. ft) • Warehouse located at ground floor level with offices delivery & reception area. There are also offices at first floor level. • Located on Railway Road in Charleville, Co. Cork. • On site car parking available to the front of the property. Tel: (061) 318 770 Web: www.powerandassociates.com Ballysallagh Industrial Estate, Charleville, Co. Cork LOCATION Charleville is one of the largest towns in North cork and acts as a growth development centre for a large rural hinterland both in County Cork and Limerick. It is strategically situated on the N20 Cork/Limerick National primary Route and the town is served by a train station on the Cork/Dublin Railway line. Charleville is situated approximately 60KM north of Cork City and 40 KM south of Limerick City. The subject property is situated approximately 1.5 km south east of Charleville Town Centre in Ballysallagh Industrial Estate. The Industrial Estate is accessed off Station Road which leads directly to Charleville Railway Station. DESCRIPTION The subject property comprises a detached modern warehouse premises which was constructed approximately 15 years ago. The unit is of steel frame construction covered by a double skin metal deck roof. The walls have external clad elevations with the front reception area comprising ab rick finish. Internally, the property comprises main warehouse area incorporating roller shutter rolling door at its southern elevation and two storey office/staff facilities section to the front. -

Bandon Legal Town and Its Environs Co. Cork
AREA PROFILE FOR TOWN BANDON LEGAL TOWN AND ITS ENVIRONS CO. CORK AGE/SEX In April 2011 Bandon had a population of 6,640, consisting of 3,276 males and 3,364 females. The population of pre-school age (0-4) was 560, of primary school going age (5-12) was 683 and of secondary school going age (13-18) was 511. There were 731 persons aged 65 years and over. The number of persons aged 18 years or over was 4,967. MARITAL STATUS Of the 5,231 persons aged 15 years and over, 2,211 were single, 2,396 were married, 171 were separated, 156 were divorced and 297 were widowed. LIVING ARRANGEMENTS There were 2,542 private households in Bandon in April 2011, of which 676 were single person households. Of the 1,769 families in the area, 528 were couples with no children. The average number of children per family was 1.3 compared with 1.4 nationally. HOUSEHOLDS BY COMPOSITION Bandon State No. of households % breakdown % breakdown One Person 676 26.6 23.7 Couple without children 494 19.4 18.9 Couple with children 830 32.7 34.9 Lone parent family 314 12.4 10.9 Other 228 9.0 11.6 Total 2,542 100.0 100.0 MIGRATION AND NATIONALITIES 92.6 per cent of the usually resident population aged over 1 lived at the same address one year before the census. A further 5.6 per cent lived elsewhere in the same county, 0.4 per cent lived elsewhere in the State while 1.3 per cent lived outside the State twelve months before the census on April 10, 2011. -

Cork County Council Energywatchit Brings €80K Annual Savings to Council IT Energy Bills
Cork County Council EnergyWatchIT brings €80k annual savings to Council IT energy bills. Pictured at the EnergyWatchIT Launch in the Council Chamber, County Hall (From left) Sean Cronin, CEO, 3 Pro EnergyWatch Ltd, Ian O’Driscoll, Director 3Pro Energy Watch Ltd, Jim Mulcahy, Director 3Pro Energy Watch Ltd, Cllr Tim Lombard, Mayor of County Cork, Martin Riordan, County Manager, Stephen Clarke, Business Development Manager, 3Pro Energy Watch Ltd, Declan Connolly, Project Coordinator ICT Department, Cork County Council, John Doherty, Software Developer, 3Pro Energy Watch Ltd Annual savings in excess of €80,000 and a reduction of C02 emissions by at least 215,000KG per annum can be achieved by Cork County Council following its involvement in the development of energy saving technology for personal computers. Cork County Council’s ICT Department began collaborating closely with the EnergyWatchIT team over a year and a half ago when it was still in UCC’s Incubation Centre. Launching the EnergyWatchIT initiative, Cllr Tim Lombard, Mayor of County Cork said, “The Council takes its environmental responsibilities very seriously and we have now adopted EnergyWatchIT as our flagship project for the new Energy Map partnership process signed with the Sustainable Energy Authority of Ireland (SEAI). “We expect to save in excess of 400,000kWh of electricity p.a. which will contribute greatly to our National target of a 33% saving in energy by 2020. We will also reduce our C02 emissions by at least 215,000 kg p.a. aiding our commitments of a 21% reduction -

The Cork to Passage Railway
THE PROPOSED CORK TO PASSAGE RAILWAY 1837 Brendan Hall The Parliamentary Committee to consider the Bill for the construction of a railway between Cork and Passage West met over three days in April 1837. The members of the Committee were Mr. O'Connell, Mr. D. Callaghan1, Mr. Jephson, Mr. Serjeant Jackson, Mr. Longfield2, Mr. Roche3, Mr. Vesey, Sir Andrew Agnew, Sir R. Bateson, Mr. Barry4, Mr. Cole, Colonel Thomas, Mr. E. Buller and Sir Richard Musgrave. There were several petitions against the Bill. One was from a group of Cork (city and county) inhabitants, alleging that the proposed railway would be harmful to the navigation of the river Lee and to trade in Cork city. No evidence was put forward to support these allegations and the claim was rejected. A petition forwarded by a consortium interested in constructing a competing railway service between Cork and Cove was also rejected. The Committee looked sympathetically on a protest by William Wise, on whose land it was proposed to build a railway terminus on the side of the river Lee. Traffic from Cork to Passage (1837) Statement showing the present annual amount of traffic, and the expected annual traffic by the Cork and Passage Railway, in Passengers and Goods - Present avg. Expected Average Amount of amount of annual charge receipts passengers traffic by by expected annually Railway Railway annually To and from Cork to Passage: Passengers by public vehicles 267,000 524,000 9d £18,650 Passengers by private vehicles 100,000 150,000 9d £5.625 Goods from Cork to Passage Dead stock, in tons 6,000 20,000 1s 6d £1,500 Live stock, pigs and sheep - 50,000 3d £625 In 1837 the average time taken to make the journey between Cork and Passage was one hour for those travelling by 'car' and around nine hours for the transportation of goods by carriers; vessels took anything between four hours and five days, depending on the state of the winds and tides.