Assessment Report on Lavandula Angustifolia Miller, Aetheroleum and Lavandula Angustifolia Miller, Flos
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
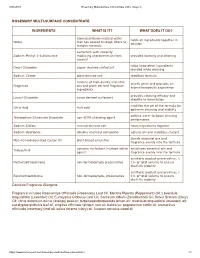
ROSEMARY MULTI-SURFACE CONCENTRATE INGREDIENTS WHAT IS IT? WHAT DOES IT DO? Contains Fragrance Allergens Fragrance Includes Rosm
9/24/2019 Rosemary Multi-Surface Concentrate | Mrs. Meyer's ROSEMARY MULTI-SURFACE CONCENTRATE INGREDIENTS WHAT IS IT? WHAT DOES IT DO? deionized/demineralized water holds all ingredients together in Water that has passed through filters to solution remove minerals surfactant with viscosity Sodium Methyl 2-Sulfolaurate modifying characteristics from provides foaming and cleaning coconut helps keep other ingredients Decyl Glucoside sugar- derived surfactant blended while cleaning Sodium Citrate plant-derived salt stabilizes formula mixture of high quality essential smells great and provides an Fragrance oils and plant-derived fragrance aromatherapeutic experience ingredients provides cleaning efficacy and Lauryl Glucoside sugar derived surfactant stability to formulation modifies the pH of the formula for Citric Acid fruit acid optimum cleaning and stability softens water to boost cleaning Tetrasodium Glutamate Diacetate non-EDTA chelating agent performance Sodium Sulfate mineral-derived salt holds ingredients together Sodium Hydroxide alkaline chemical compound adjusts pH and stabilizes chelant blends essential oils and PEG-40 Hydrogenated Castor Oil plant based emulsifier fragrance evenly into the formula nonionic surfactant (surface active solubilizes essential oils and Trideceth-9 agent) fragrance evenly into the formula synthetic product preservative, < Methylisothiazolinone non-formaldehyde preservative 1% of total volume to ensure shelf life stability synthetic product preservative, < Benzisothiazolinone non-formaldehyde, preservative -

Sharp's at Waterford Farm Your Neighborhood Farm Ask Us How To
Lemongrass – Essential for Thai Sharp’s at Waterford Herbs List cooking Farm Anise - Hyssop Lovage (Levistcum officinale) Farming in Howard County Basil Marjoram (Origanum majorana) since 1903 African Blue Amethyst Improved Purple Sweet Eleonora Zaatar, a hint of thyme, oregano & 4003 Jennings Chapel Rd. Elidia - Compact; container basil marjoram Brookeville, MD 20833 Genovese Golden - ornamental mostly Holy - Sacred Red and Green Tel: (410) 489-2572 Mint (Mentha sp.) Italian Large Leaf Chocolate Peppermint Lemon – Mrs. Burns www.sharpfarm.com Lemon Mint Mountain Mint Lettuce Leaf – Napoletano email: Peppermint Pineapple Mint Lime [email protected] Spearmint Sweet Thai Dark Opal Oregano (Origanum sp.) Red Rubin Greek Rutgers Devotion Zaatar ( a hint of thyme, oregano, & marjoram) Oreganum Syriaca) Borage: the herb of gladness Hot and Spicy - real tang, our favorite for adding to beans Catnip (Nepeta)- feline friends treat Parsley (Petroselinum crispum) Calendula, Neon Plain leaf (Italian or flat) Curly – double or triple Chamomile (German) Organic curled parsley (Bodegold) Italian Dark Green – Giant of Italy – huge leaves Your Neighborhood Chervil (Anthricus cerefolium) ‘crispum’ Vertissimo Farm Rosemary (Rosmarinus) Arp Chives (Allium) Hill Hardy Med Leaf (Purly) Ask Us How to Garden Salem Large leaf (staro) Sage (Salvia offincinalis) Helpful Hints: We pride ourselves Cilantro (Coriandrum sativium) Garden - Extrakta on knowing how to vegetable and herb Cruiser – more upright – great for Pineapple garden. Please ask if you need bunching – 50 days Savory Winter information on how to. Yields? Cutting Celery (Apium graveolens) Sorrel, French Spacing between plants? Staking? aka leaf celery When you plant, space your harvest Stevia (Stevia rebaudiana) by using varieties of different maturity Dill (Anethum graveolens): Nature’s natural sweetener dates. -

Medicinal Plants, Aromatic Herbs and Fragrance Plants in France
> Medicinal plants, aromatic herbs and fragrance plants in France: a small www.ihc2022.org but thriving sector with a strong traditional base and a dynamic research network Annabelle Bergoënd and Joséphine Piasentin In France, medicinal and aromatic plants paced growth. This production is very diverse small areas. Many producers reserve some (MAP) or herbal, medicinal and aromat- and dynamic, leaning on strong traditional areas as a side crop for their main produc- ic plants (HMAP) industries1 traditionally skills and benefiting from new techniques tion. Many of the plants are not referenced refer to culinary herbs, medicinal plants, and high value processing. With a growing in the European common agricultural pol- and plants cultivated for the fragrance and demand for natural products and unfolding icy (CAP) nomenclature. About 75% of the perfume industries. This agricultural sector opportunities for new markets, HMAP is fac- producers cultivate HMAPs to diversify their encompasses several hundred plant species, ing exciting prospects. production. The largest amount of land is as compared with field crops, such as cereals, occupied by fragrance plants, then medici- which may include single species grains. Characteristics of French HMAP nal plants. Aromatic herbs account for less The organic production for these crops is than 10% of the HMAP cultivated area. In significantly larger than traditional crops of Farm features and production are 2018, the estimated average farm was 4 ha global French agriculture. Wild harvest of geographically diverse for MAP production and 17 ha for perfume HMAP continues and is key for a success- The complexity of the production sector for plants. -

Antibacterial Effects of Citrus Limon and Lavandula Angustifolia Essential Oils
Journal of Applied Biological Sciences E-ISSN: 2146-0108 14(3): 358-364, 2020 Research Article ANTIBACTERIAL EFFECTS OF CITRUS LIMON AND LAVANDULA ANGUSTIFOLIA ESSENTIAL OILS Naima Sahraoui1, Meriem Djeghboub2*, Khelaf Saidani1, Souad Djeghboub1, 2 Jean Luc Hornick 1Veterinary sciences institute of Blida 1 university, Algeria PO box 270, Soumaa road, Blida – Algeria 2Department of Animal Resources Management, Faculty of Veterinary Medicine, University of Liège, FARAH Center, Quartier Vallée 2, Avenue de Cureghem 6, B43a, 4000 Liège, Belgium *Corresponding Author: E-mail: [email protected] (Received 14th January 2020; accepted 06th April 2020) ABSTRACT. Essential oils have been largely used for their antibacterial, antifungal and insecticidal properties. Thus this study evaluated the in vitro antibacterial activity of essential oils from lemon and lavender on 30 bacterial strains of Escherichia coli and Staphylococcus aureus either isolated from animals (cows and sheep) or obtained from reference strains provided by the Biotechnology and Animal Reproduction from the Laboratory of the Institute of Veterinary Sciences from Blida. The sensitivity of the strains was tested in vitro by aromatogram, using the two essential oils separately as well as their associations. Our results showed that lemon essential oil had a bacteriostatic effect on the tested strains of E. coli, while a high percentage of them was extremely sensitive to lavender oil, and more than half ones were extremely sensitive to the oil blend at a concentration of 100µl/disc. As for S. aureus, all the tested strains were extremely sensitive to the two essential oils as well as to their association. Lavender and/or lemon essential oils proved effective against strains of E. -

The Chromosome-Based Lavender Genome Provides New Insights Into
Li et al. Horticulture Research (2021) 8:53 Horticulture Research https://doi.org/10.1038/s41438-021-00490-6 www.nature.com/hortres ARTICLE Open Access The chromosome-based lavender genome provides new insights into Lamiaceae evolution and terpenoid biosynthesis Jingrui Li1,2, Yiming Wang 3,YanmeiDong1,2, Wenying Zhang1,2,DiWang1, Hongtong Bai1,KuiLi3,HuiLi1 and Lei Shi1 Abstract The aromatic shrub Lavandula angustifolia produces various volatile terpenoids that serve as resources for essential oils and function in plant-insect communication. To better understand the genetic basis of the terpenoid diversity in lavender, we present a high-quality reference genome for the Chinese lavender cultivar ‘Jingxun 2’ using PacBio and Hi-C technologies to anchor the 894.50 Mb genome assembly into 27 pseudochromosomes. In addition to the γ triplication event, lavender underwent two rounds of whole-genome duplication (WGD) during the Eocene–Oligocene (29.6 MYA) and Miocene–Pliocene (6.9 MYA) transitions. As a result of tandem duplications and lineage-specific WGDs, gene families related to terpenoid biosynthesis in lavender are substantially expanded compared to those of five other species in Lamiaceae. Many terpenoid biosynthesis transcripts are abundant in glandular trichomes. We further integrated the contents of ecologically functional terpenoids and coexpressed terpenoid biosynthetic genes to construct terpenoid-gene networks. Typical gene clusters, including TPS-TPS, TPS- CYP450, and TPS-BAHD, linked with compounds that primarily function as attractants or repellents, were identified by fl 1234567890():,; 1234567890():,; 1234567890():,; 1234567890():,; their similar patterns of change during ower development or in response to methyl jasmonate. Comprehensive analysis of the genetic basis of the production of volatiles in lavender could serve as a foundation for future research into lavender evolution, phytochemistry, and ecology. -

Chemical Composition and Acaricidal Effects of Essential Oils of Foeniculum Vulgare Mill
Hindawi Publishing Corporation Psyche Volume 2014, Article ID 424078, 6 pages http://dx.doi.org/10.1155/2014/424078 Research Article Chemical Composition and Acaricidal Effects of Essential Oils of Foeniculum vulgare Mill. (Apiales: Apiaceae) and Lavandula angustifolia Miller (Lamiales: Lamiaceae) against Tetranychus urticae Koch (Acari: Tetranychidae) Asgar Ebadollahi,1 Jalal Jalali Sendi,1 Alireza Aliakbar,2 and Jabraeil Razmjou3 1 Department of Plant Protection, Faculty of Agricultural Sciences, University of Guilan, Rasht 416351314, Iran 2Department of Chemistry, Faculty of Basic Sciences, University of Guilan, Rasht, Iran 3Department of Plant Protection, Faculty of Agriculture, University of Mohaghegh Ardabili, Ardabil, Iran Correspondence should be addressed to Asgar Ebadollahi; [email protected] Received 17 September 2014; Revised 14 November 2014; Accepted 24 November 2014; Published 14 December 2014 Academic Editor: Nguya K. Maniania Copyright © 2014 Asgar Ebadollahi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Utilization of synthetic acaricides causes negative side-effects on nontarget organisms and environment and most of the mite species such as two spotted spider mite, Tetranychus urticae Koch, are becoming resistant to these chemicals. In the present study, essential oils of fennel, Foeniculum vulgare Mill., and lavender, Lavandula angustifolia Miller, were hydrodistilled using Clevenger apparatus and chemical composition of these oils was analyzed by GC-MS. Anethole (46.73%), limonene (13.65%), and -fenchone (8.27%) in the fennel essential oil and linalool (28.63%), 1,8-cineole (18.65%), and 1-borneol (15.94%) in the lavender essential oil were found as main components. -

Composition and Antimicrobial Activity of Some Essential Oils from Serbia
FOOD ANALYSIS LJUBIŠA ŠARIĆ1*, IVANA ČABARKAPA1, BOJANA ŠARIĆ1, DRAGANA PLAVŠIĆ1, JOVANKA LEVIĆ1, SAVA PAVKOV2, BOJANA KOKIĆ1 *Corresponding author 1. University of Novi Sad, Institute of Food Technology, Bulevar cara Lazara 1, Novi Sad, 21000, Serbia 2. Institute for Medicinal Plant Research, Dr. Josif Pančić, Tadeuša Košćuška 1, Belgrade, 11000, Serbia Ljubiša Šarić Composition and antimicrobial activity of some essential oils from Serbia KEYWORDS: essential oils, chemical composition, antimicrobial activity The aim of this work was to determine chemical composition and antimicrobial properties of essential oils of AbstractRosmarinus officinalis L., Salvia officinalis L, Lavandula angustifolia L. and Mentha piperita L. originated from Serbia. The main components of rosemary essential oil were camphor (17.66 %), 1,8 cineole (16.11 %), verbenone (13.84 %), α-pinene (12.45 %) and borneol (9.22 %). Sage essential oil contained 28.64 % of camphor 21.90 % of 1,8 cineole and 19.92 % of α-thujone (16.92 %). Camphor (17.14 %), 1,8 cineole (12.74 %) and linalyl acetate (9.62 %) were the most abundant compounds of lavander essential oil. Concentrations of menthol and menthone as the main compounds of mint essential oil were 38.82 % and 26.12 %, respectively. All essential oils showed antimicrobial activity against all tested bacteria in the MIC range of 0.16 – 5.00 mg/ml and MBC range of 0.63 – 5.00 mg/ml. INTRODUCTION and diseases. In the popular medicine, it is used to treat nausea, flatulence, vomiting, indigestion, stomach Increased demand for natural and safe food, without cramps, menstrual cramps and parasitosis. It is also chemical preservatives, provokes many researches to recognized for its carminative, stimulant, antispasmodic, investigate the antimicrobial effects of natural antiseptic, anti-inflammatory, antibacterial and antifungal compounds. -

ABS POMEGRANATE STEROLS Gloss Effect + Intense Moisturization + Skin Barrier Benefits + Hair and Skin Care
ABS POMEGRANATE STEROLS Gloss Effect + Intense Moisturization + Skin Barrier Benefits + Hair and Skin Care Tomorrow’s Vision… Today!® PRODUCT REFERENCES ABS Pomegranate Sterols Product Code: 10247 INCI Name: Punica Granatum (Pomegranate) Sterols INCI Status: Approved Suggested Use Levels: 0.5 – 5.0% Suggested Applications: Moisturization, Hydration, Enhanced Barrier Function, Color Cosmetic Applications, Hair and Skin Care Solubility: Oil Soluble Who is using Pomegranate Sterols? • ALMAY • CLARINS • BARE MINERALS • AVEDA ALMAY: Pure Blends Lip Gloss Product Description: Lightweight formula glides on smoothly for a natural finish. Pure Blends Lip Gloss by Almay incorporates a blend of natural antioxidants including lotus, orchid and acai. Packaging is made from 35.0% recycled materials! Ingredients: Sweet Almond Oil -PrunusAmygdalusDulcis, PunicaGranatumSterols , DisteardimoniumHectorite, Beeswax - CeraAlba , Jojoba Seed Oil -SimmondsiaChinensis, AcaiFruit Extract -EuterpeOleracea, YuzuPeel Extract -Citrus Junos, Lotus Flower Extract -NelumboNucifera, Orchid Flower Extract -Cymbidium Grandiflorum, Wild Pansy Extract -Viola Tricolor , Papaya Fruit Extract -CaricaPapaya , Orange Oil -Citrus AurantiumDulcis, Vanilla PlanifoliaFruit , LitseaCubebaFruit Oil , SorbicAcid , Mica -May Contain , Titanium Dioxide -CI 77891 -May Contain , Iron Oxides -CI 77491 -77492 -77499 -May Contain , Yellow 6 -CI 15985 -May Contain , Red 6 -CI 15850 -May Contain , Red 7 -CI 15850 -May Contain , Carmine -CI 75470 CLARINS: Instant Gloss Magic Color Product Description: -

Apium Graveolens, Nux Vomica Oil Healing Natural Oils
H-HEADACHES FORMULA- apium graveolens, nux vomica oil Healing Natural Oils LLC Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective. ---------- Active Ingredients: Apium graveolens - 12C HPUS Nux vomica -12C HPUS Purpose Apium graveolens - 12C HPUS (throbbing headaches), Nux vomica -12C HPUS (headache in occiput or over eyes) The letters ‘HPUS’ indicate that the components in this product are officially monographed in the Homoeopathic Pharmacopoeia of the United States Inactive Ingredients: Corylus avellana nut oil, Essential Oil Blend (Citrus limon peel, Lavandula officinalis flower bud, Matricaria recutita flower, Mentha piperita flower and whole plant, Rosmarinus officinalis whole plant). Indications: For the temporary relief of headache symptoms such as throbbing pain, pain above the eyes. Directions: Adults and Children 4 yrs and over: Wash hands before and after use. Shake well and apply 1 or 2 drops to fingers and massage temples and back of neck every 30mins or when needed, no more than 5x per day. Do not oversaturate or exceed dosage. Warnings: For external use only. Perform skin test prior to initial use. Do not use if pregnant or breastfeeding. Keep out of reach of children. Keep away from eyes and mucous membranes. If swallowed get medical help or contact a Poison Control Center right away. Discontinue use if irritation occurs. Consult a doctor if symptoms worsen after 7 days. Dist. by Healing Natural Oils 3830 Valley Ctr Dr, #705-526, San Diego, CA 92130, USA Healing Natural Oils H-Headaches Formula Homeopathic For External Use Only 038 11ml (0.37 fl. -

Product Name Ingredients
Product Name Ingredients Cream Cleanser Matricaria chamomilla (recutita - Chamomile) Extract*, Aloe barbadensis (Aloe Vera ) Leaf juice*, Olivoyl Hydrolyzed Wheat Protein, Cetearyl Alcohol, Glyceryl Oleate, Glyceryl Stearate, Glycerin, Macadamia integrifolia (Macadamia) Seed Oil*, Stearic Acid, Dicaprylyl Ether, Morinda citrifolia (Noni Fruit) Extract*, Persea gratissima (Avocado) Oil*, Rosa eglentaria (Rose Hip) Oil*, Tocopherol (Natural Vitamin E), Helianthus annuus (Sunflower) Oil, Xanthan Gum, Benzyl Alcohol, Dehydroacetic Acid, Citrus aurantium ssp.bergamia (Bergamot) Oil*, Santalum spicatum (Sandalwood) Oil*, Citrus reticulata (Mandarin)Oil*, Citrus paradisi (Grapefruit) Oil*, Lavandula angustifolia (Lavender) Oil*, Ascorbyl Palmitate Lecithin (GMO free)*, Potassium Hydroxide, Lactic Acid, Sodium Chloride (Macrobiotic sea salt solution), Beta Carotene, Olea europaea (Olive) Oil, Aqua (Water), limonene**. *Ingredient from organic farming. **From essential oils. 98.33% of the total ingredients are from Natural Origin. 50.71% of the total ingredients are from Organic Farming. Foaming Cleanser Camellia sinensis (Green Tea)*, Rosa centifolia (Rose) Extract*, Aloe barbadensis (Aloe Vera) Leaf juice*, Coco-glucoside, Sodium Cocoyl Glutamate, Glycerin, Xanthan Gum, Glyceryl Oleate, Morinda citrifolia (Noni Fruit) Extract*, Benzyl Alcohol, Dehydroacetic Acid, Santalum spicatum (Sandalwood) Oil*, Cedrus atlantica (Cedarwood) Oil*, Dicaprylyl Ether, Lecithin (GMO free)*, Lactic Acid, Sodium chloride (Macrobiotic sea salt), Aqua (Water). *Ingredient -

Antibacterial Effect of Essential Vegetal Extracts on Staphylococcus Aureus Compared to Antibiotics
Available online at www.notulaebotanicae.ro Print ISSN 0255-965X; Electronic ISSN 1842-4309 Not. Bot. Hort. Agrobot. Cluj 37 (2) 2009, 117-123 Notulae Botanicae Horti Agrobotanici Cluj-Napoca Antibacterial Effect of Essential Vegetal Extracts onStaphylococcus aureus Compared to Antibiotics Iosif Nicodim FIT, Gheorghe RAPUNTEAN, Sorin RAPUNTEAN, Flore CHIRILA, George Cosmin NADAS University of Agricultural Sciences and Veterinary Medicine, Faculty of Veterinary Medicine, 3-5 Manastur Street, 400372, Cluj-Napoca, Romania; [email protected] Abstract This study is aiming to evaluate the antimicrobial activity of ten essential vegetal extracts on strains of Staphylococcus aureus isolated from animal lesions. The comparative effect of some antibiotics on these strains isolated from animals was also tested and estimated. The extracts were represented by Albies alba, Aloe barbadensis, Calendula officinalis, Cocos nucifera, Eucalyptus globulus, Hypericum perforatum, Lavandula angustifolia, Satureja hortensis, Mentha piperita, Pinus silvestris essential oils, in three amounts (30 μl, 3 μl and 0,3 μl) and comparatively eight antibiotics frequently utilized in staphylococci infections treatment in animals were used. Animal origin S. aureus strains were identified using API staph tests (BioMerieux, France). The tests were realized by diffusion method using Muller Hilton agar while the antibacterial effect was interpreted depending on the inhibition area diameter. The obtained results revealed that Satureja hortensis and Albies alba extracts inhibit the develop of the most staphylococci tested strains. The highest inhibition areas were observed for the amounts of 30 μl and 3 μl essential extract. Most of the staphylococci strains were resistant to antibiotics, the most efficient being Ceftiofur and Methicillin. The results are suggesting that savory and fir has antibacterial effect onS. -

Topical Emulsion Containing Lavandula Stoechas Essential Oil As a Therapeutic Agent for Cutaneous Wound Healing
Article Topical Emulsion Containing Lavandula stoechas Essential Oil as a Therapeutic Agent for Cutaneous Wound Healing Mohamed Nadjib Boukhatem 1,2,* , Henni Chader 3,4, Aicha Houche 1, Faiza Oudjida 5, Fatma Benkebaili 1 and Yahia Hakim 6 1 Department of Biology, Faculty of Life and Natural Sciences, University Blida 1, Blida 09000, Algeria; [email protected] (A.H.); [email protected] (F.B.) 2 Laboratoire Ethnobotanique et Substances Naturelles, Ecole Normale Supérieure, Kouba, Alger 16047, Algeria 3 Laboratoire Pharmaco-Toxicologie, Laboratoire National de Contrôle des Produits Pharmaceutiques (LNCPP), Dély Brahim, Alger 16047, Algeria; [email protected] 4 Faculté de Médecine, Université Ben Youcef Ben Khedda, Alger I, Alger 16000, Algeria 5 Laboratoire Anatomie Pathologique, Centre Hospitalo-Universitaire de Beni Messous, Alger 16206, Algeria; [email protected] 6 Extral-Bio Company, Chiffa, Blida 09000, Algeria; [email protected] * Correspondence: [email protected]; Tel.: +213-664983174 Abstract: Background and objectives: The present research was designed to evaluate the chemical composition of Lavandula stoechas essential oil (EOLS) as well as the in vivo wound-healing property. The chemical composition of EOLS was identified by gas chromatography mass spectrometry. Nine- teen compounds of EOLS were reported. Linalool was identified as the major chemical compound Citation: Boukhatem, M.N.; (24.87%), followed by linalyl acetate (19.10%). EOLS showed a high content of oxygenated com- Chader, H.; Houche, A.; Oudjida, F.; pounds (63.54%). In vivo wound healing activity of the topical cream prepared from EOLS (0.5% w/w) Benkebaili, F.; Hakim, Y. Topical was assessed using a circular excision wound model.