Download Article (PDF)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Special Notes and Students Learning Contents During Lock Down Period: Prepared By: Dr
Special Notes and students learning contents during Lock down period: Prepared by: Dr. M.K.Saikia Associate Professor & Coordinator BTHub Dhing College: Nagaon( Assam) B.Sc II Semester BOTANY ( Major) Unit 9: Notes on Applied Mycology. Q. How can you describe about the Potential application of Fungi in Biotechnology: [Students can read or go through the following hints/points] Fungi are prominent sources of pharmaceuticals and are used in many industrial processes such as the production of enzymes, vitamins, pigments, lipids, glycolipids, polysaccharides and polyhydric alcohols. During the past 50 years, several major advancements in medicine came from lower organisms such as molds, yeasts and the other diver's fungi. Fungi are extremely useful in making high value products like mycoproteins and acts as plant growth promoters and disease suppressor. Fungal secondary metabolites are important to our health and nutrition and have tremendous economic impact. In addition to this, fungi are extremely useful in carrying out biotransformation processes. Recombinant DNA technology, which includes yeasts and other fungi as hosts, has markedly increased market for microbial enzymes. Today, fungal biotechnology is a major participant in the global industry due to its mind-blowing potential. 1. Fungi in Designing of vectors: Yeast vectors are used in genetic engineering. E.g., shuttle vectors are used for expression of desirable gene in both prokaryotic and eukaryotic systems. YAC, YRP, YIP, YEP are some other yeast vectors. 2)Fungi as a food: Fungi are used as high cost food because of its high protein and low calorific value. Europe, America, Australia and Japan are very playing industries in mushroom cultivation. -

Cancer Research in the Far East E
Postgrad Med J: first published as 10.1136/pgmj.37.428.360 on 1 June 1961. Downloaded from POSTGRAD. MED. J. (ig6I), 37, 360 CANCER RESEARCH IN THE FAR EAST E. BOYLAND, D.Sc. Professor of Biochemistry, Institute af Cancer Research, Royal Cancer Hospital, Fulham Road, London, S.W.3 WITH the recognition of the widespread incidence liver occur more frequently in Japan than in of cancer, research into its occurrence, cause and western countries; the elucidation of the causes of treatment is being pursued in an increasing these forms of cancer presents important, interest- number of centres. This research is supported ing, but difficult problems which are being by governments, by industry and by private studied. The incidence of these forms of cancer subscription. The biggest effort is undoubtedly appears to be influenced by diet, but the relation- being made in the United States of America ship between diet and cancer in man remains where very large sums of money are being spent, obscure. One of the dietary factors suspected as particularly in the field of chemotherapy of possibly responsible for cancer in man is yellowed cancer; this is financed mainly by the government or mouldy rice. One of the moulds which is United States Health Service and the American commonly present in rice stored in damp condi- Cancer Society which relies on private donations. tions is Penicillium islandicum. Feeding of rice One had the impression that the support of artificially infected with the penicillium induced a cancer research in Japan was to some extent few tumours in rats. -

Project of Fact Finding Survey for Poverty Reduction and Sustainable Agricultural Development in Myanmar
Project of Fact Finding Survey for Poverty Reduction and Sustainable Agricultural Development in Myanmar Project Report March 2017 Japan Association for International Collaboration of Agriculture and Forestry JAICAF Japan Association for International Collaboration of Agriculture and Forestry Akasaka KSA Bldg 3F, 8-10-39, Akasaka, Minato-ku, Tokyo 107-0052, JAPAN Tel: 81-3-5772-7880 ISBN: 978-4-908563-16-4 print ISBN: 978-4-908563-17-1 pdf Foreword Japan Association for International Collaboration of Agriculture and Forestry, JAICAF, implemented this project with financial support from the Ministry of Agriculture, Forestry and Fisheries of Japan aiming at prevention of post-harvest loss of rice in Myanmar. One of the most important staple crop for Myanmar is rice, which occupies more than half of farmland area. Myanmar government is said to aim at increase of export and quality improvement of rice. Based on this background, we have been dispatching experts to Myanmar since Fiscal Year 2014 in order to cooperate for the sustainable development of rice production and post-harvest processing. For the past two years, we have been actively working on the improvement of post-harvest handling of rice mainly in Naypyidaw area. This year, as well as to continue our activities in Naypyidaw, we decided to disseminate our project achievement to other regions such as Ayeyarwady region. This report describes the activities and results of the project in FY 2016. We hope that this report will be useful to other rice projects in Myanmar. The project would not have succeeded without support and advice of the dispatched experts and member of Evaluation and Review Committee. -

Breeding of Selenium Rich Red Glutinous Rice, Protein Extraction and Analysis of the Distribution of Selenium in Grain
INTERNATIONAL JOURNAL OF AGRICULTURE & BIOLOGY ISSN Print: 1560–8530; ISSN Online: 1814–9596 17–1103/2018/20–5–1005–1011 DOI: 10.17957/IJAB/15.0592 http://www.fspublishers.org Full Length Article Breeding of Selenium Rich Red Glutinous Rice, Protein Extraction and Analysis of the Distribution of Selenium in Grain Yuanke Liang1†, Muhammad Umer Farooq1†, Rui Zeng1†, Zhicheng Tang1, Yujie Zhang1, Tengda Zheng1, Hla Hla Ei1, Xiaoying Ye1, Xiaomei Jia1 and Jianqing Zhu1*† 1Rice research institute, Sichuan Agricultural University, Chengdu, Sichuan 611130, China *For correspondence: [email protected] †These authors contributed equally to this work Abstract Selenium acts as a chemo preventive agent for specific cancers, which can be useful as protective agent against stress. The objective of this study was to breed healthy selenium rich red glutinous rice serving as diet and search for convenient methods to extract protein from grain and measure the protein and selenium concentration. Furthermore, determine the interaction of selenium and protein in different parts of red glutinous rice seeds. After progress of five years breeding, the red glutinous rice achieved the standard (≥40 ng/g) selenium contents of 121.75 ± 3.01 ng/g. Total selenium concentrations in the rice fractions varied in the following order: husk ˂ polished rice ˂ whole grain ˂ bran. Through the analysis of the protein content in different red glutinous rice parts, compared with the selenium concentration, we have made a conclusion that more than 80% selenium in the grain is organic selenium (in selenoprotein form) and more than half of the total selenium is in the endosperm of the red glutinous rice. -

Physicochemical , Morphological, Pasting and Sofetening Kinetic Properties of Selected Rice Varieties from West-Africa
PHYSICOCHEMICAL , MORPHOLOGICAL, PASTING AND SOFETENING KINETIC PROPERTIES OF SELECTED RICE VARIETIES FROM WEST-AFRICA by Chijioke Anthony Nwankpa Department of Food Science and Agricultural Chemistry MacDonald Campus, McGill University Montreal, Quebec. A thesis submitted to the Faculty of Graduate Studies and Research in partial fulfillment of the requirements for the degree of Master of Science. © Chijioke Anthony Nwankpa, 2013 i Suggested Short Title Characterization of rice varieties from West Africa ii FOREWORD This thesis is reported in the form of original papers expected to be published in journals. The first two sections of this study comprises of a general introduction presenting a brief review of the literature on the topic s in addition to the rationale and objectives behind the study. The next two sections make up the body of the thesis with each chapter representing a complete manuscript, while the last chapter is a summary of the major conclusions. This format has been approved by the Faculty of Graduate Studies and Research, McGill University in accordance with the conditions provided in the Guidelines for Thesis Preparation, Thesis Specification, section 3 entitled 'Traditional and manuscript-based theses" which as follows: " Candidates have the option of including, as part of the thesis, the text of a paper(s) submitted or to be submitted for publication, or the clearly duplicated text of a published paper(s). These texts must be bound as an integral part of the thesis. If this option is chosen, connecting texts that provide logical bridges between the different papers are mandatory. The thesis must be written in such a way that it is more than a mere collection of manuscripts; in other words, results of a series of papers must be integrated. -

Mold Toxins: Hazard to Animal and Human Health
Mold toxins: Hazard to animal and human health Brunhilde Kobbe Mold toxins or mycotoxins are new the peanut meal used as feedstuff. When aflatoxin in urine samples is being devised. “in” words among microbiologists and tox- the new toxins turned out also to be potent Detection of aflatoxin or its metabolic pro- icologists and may soon also be familiar to carcinogens, and the Aspergillus fungus to ducts could provide valuable information the general public. At times we all have en- be a common invader of foodstuffs, it be- about the metabolism of this toxin in the countered colorful layers of mold growth came apparent suddenly that the molds body, and it would be a warning about the on spoiled bread, fruit, or vegetables. Re- could also pose a great hazard to human exposure to aflatoxin and possible liver cent research has shown that these harmless health. damage. looking invaders have to be taken seriously; Meanwhile a few other carcinogenic my- Another approach to assess human expo- some produce potent toxins and carcino- cotoxins have been discovered. For exam- sure has been the use of epidemiological gens. ple, traces of patulin were extracted from studies, where scientists have looked for a Food spoilage by fungi is a familiar prob- apple juices; luteoskyrin has been identified possible correlation between the consump- lem, although it was not always recognized as one of the “Yellowed Rice Toxins.” tion of aflatoxin-contaminated foods and as such. During the Middle Ages large-scale However, the aflatoxins still remain the the incidence of liver cancer. Surveys were outbreaks of “St. -
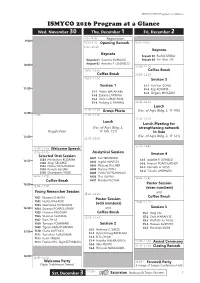
ISMYCO 2016 Program at a Glance ISMYCO 2016 Program at a Glance !"#$%&'(")*"+% !" ,-.$%/"0")*"+% # 1+2$%/"0")*"+% $
ISMYCO 2016 Program at a Glance ISMYCO 2016 Program at a Glance !"#$%&'(")*"+% !" ,-.$%/"0")*"+% # 1+2$%/"0")*"+% $ 8:00~9:00!!!!!!!!!!!!!!!!"#$%&'()'%*+ 9:00 9:00~9:10 !"#$%$&'(#)*+, 9:00~10:00 9:10~10:10 -#.$/0# -#.$/0# Keynote 03 !!"#$%&'!(")(* Keynote 01 !!)#2#3#!(45*6*7 Keynote 04 !!+,-./%-!011 Keynote 02 !!*-8%-,%!9:!0;6"71<; 10:00 10:00~10:20 10:10~10:30 1/22##'3+#*, 1/22##'3+#*, 10:20~11:20 10:30~11:50 4#55%/$'6 4#55%/$'7 S3-1 !!+#-!+#-!6;=6 11:00 S3-2 !!(%>,!*;+*5* S1-1 !!5D,E%!/*F*=*G1 S3-3 !!)?,@AB#!57+*C*(7 S1-2 !!CDEDB,D!0*F799*H S1-3 !!I,J8%B!075*+."7;) S1-4 !!1-$D-@!):!"*H*+4 11:30~12:30 89$:; 11:50~12:00 ?+/9"'@;/0/ ,-)./!*0!1$(%/!234$/!56!7-!789:! 12:00 12:00~ 12:00~13:00 11:30~13:10 89$:;' 89$:;'<##0%$&'2/+' ,-)./!*0!1$(%/!234$/!56! 50+#$&0;#$%$&'$#0=/+,' "#$%&'()'%*+ 7-!7896!757: %$'>5%* ,-)./!*0!1$(%/!234$/!56!7-!757: 13:00 13:00~15:20 13:20~14:40 13:30~13:35'''''''''''''''''B#A:/)#'4"##:; 13:35~14:35 >$*A.0%:*A'4#55%/$ 4#55%/$'C 4#A#:0#D'!+*A'4#55%/$ AS01 !!(#B8!G"4==1" SS01 !!5%8%,J?,B%!(;K*5* S4-1 !!72DNA&&A!M:!;)/*0K 14:00 AS02 !!7-@B,$!H4=F01+ SS02 !!O,-DP!)10*5*F S4-2 !!*3-DB8!M;*M;0*FH1M AS03 !!5,J?DA&!97)<H1" SS03 !!5,J?DA&!";4F01K61 S4-3 !!(A--A8?!*:!I;)) SS04 !!"L#,J?,!416*(7 AS04 !!KD-BAL!F;FH S4-4 !!FDED2?,!45154"* SS05 !!6,#2APP,-D!540 AS05 !!+#,J?,!+;F)4+*=*67 14:35~15:00 AS06 !!F?#!H4+=H 14:40~15:40 1/22##'3+#*, AS07 !!5%-,ED!M0;F*= @/50#+'4#55%/$ 15:00 15:00~17:36 'E#F#$'$9)G#+5H I/9$&'(#5#*+:;#+'4#55%/$ 15:20~16:20 )+4! 1/22##'3+#*, YS01 !!/D2D-D!<H*7)"7 @/50#+'4#55%/$ YS02 !!=%B&,D!5*H";" 15:40~17:20 -

Interaction of Nutrients and Other Factors with Mycotoxins
FA 0/U N EP/U SS P International Training Course RAINING ACTIVITIES ON FOOD CONTAMINATION CONTROL AND MONITORING WITH SPECIAL REFERENCE TO MYCOTOXINS P. M. NEWBERNE INTERACTION OF NUTRIENTS AND OTHER FACTORS WITH MYCOTOXINS Centre of International Projects, GKNT Moscow, 1985 Interaction of Nutrients and Other tactors with Myotoxins Paul N. Hewbei:ne Professor m.rLtu./5enior t.cturer Massachusetts Xnstitute of technology Cambridge1 NA 02139 and professor of Pathology oItOn Oniv.ruity School of NdLcine Introduction Throughout r.cordud history diseases of man and lower ani- male have been sa.ociat•d with metaboittes of certain molds growing on foodstuffs. Nowev.r, an understanding of the rela- tionship btween the diseases, the molds, the food and its nutrient content has begun to develop only in recent years. It has been known sinc, the turn of the century that certain fungi produce toxic metabolites eliciting biologic responses in both man and animals. A. early as the 19th century, a disease asso- ciated with the consumption of discolored 'y.11ow.d rice was recognized in Japan and established as a toxicologic .ntlty. This disease has been identified as the y.11ow*d rice syndrome. Similarly, allimantry toxic aleukia (ATA), sssoci&ted with over- wintered wheat, atf.cted both man and animal, in Russia, this disease has been known for many decades. Centuries prior to these observations, poisoning was often observed in human popule- tione, associated with the ingestion of flour and bread, later -2- ientified as being contaminated with a fungus, this created widespread epidemic, of argotism in Europe - known to the ancients as St. -

Historical Review of Researches on Yellow Rice and Mycotoxigenic Fungi Adherent to Rice in Japan
JSM JSM Mycotoxins 65(1), 19 - 23(2015) Mycotoxinsins http://dx.doi.org/10.2520/myco.65.19 Review www.jstage.jst.go.jp/browse/myco Historical review of researches on yellow rice and mycotoxigenic fungi adherent to rice in Japan Masayo Kushiro1 1Food Safety Division, National Food Research Institute, National Agriculture and Food Research Organization, 2-1-12 Kannondai, Tsukuba, Ibaraki 305-8642, Japan Keywords Abstract citreoviridin; citrinin; “Yellow rice” is the collective name of rice grains contaminated by Peni- cyclochlorotine (islanditoxin); cillum fungi in Japan. Three kinds of yellow rice, which are caused by differ- luteoskyrin; Penicillium ent sub-species of toxigenic fungi, are known. Initially, mal-nutrition resulting from Asian poverty was regarded as the source of illness in people; however, Correspondence thanks to ongoing research, the close relationship of “Shoshin-kakke (heart- Masayo Kushiro, Food Safety attacking paralysis)”, “mycotoxin”, and “rice” was elucidated. Subsequently, Division, National Food Research the illness was shown to derive from “yellow rice”, which was due to grains Institute, National Agriculture and damaged by Penicillium spp. After World War II, another two kinds of yellow Food Research Organization, 2-1-12 rice, “Islandia yellow rice” and “Citrinum yellow rice”, were found, and the Kannondai, Tsukuba, Ibaraki causative toxic compounds were determined through interdisciplinary col- 305-8642, Japan. laborative studies. This article summarized the researches on toxigenic fungi E-mail: [email protected] adherent to rice and three kinds of yellow rice identified in Japan. (Received December 20, 2014, accepted January 30, 2015) domestic rice. Introduction In 1934, the Rice Utilization Institute, a predecessor Rice is the primary staple food for Japanese. -

Toxic Effects of Citrinin in Animals and Poultry
Vol.5 No.4 April-June 2018 ISSN: 2321-6387 Toxic Effects of Citrinin in Animals and Poultry D.Basheer Ahamad Department of Veterinary Pathology, Veterinary College and Research Institute, Tamil Nadu Veterinary and Animal Sciences University, Tirunelveli, Tamil Nadu, India Article Received on 20.03.2018 Article Published on 19.04.2018 Introduction and Aspergillus oryzae, the latter being Citrinin (CTN) is a secondary used to produce sake, miso, and soy sauce product of fungal metabolism, first isolated (Manabe, 2001). by Hetherington and Raistrick from a Citrinin is nephrotoxic and culture of Penicillium citrinum Thom implicated in disease outbreaks in both (Hetherington and Raistrick, 1931). animals and humans. Acutely lethal doses Meanwhile, several other fungal species administered to rabbits, guinea pigs, rats, within the three genera, Penicillium (P. swine, or mice caused swelling of the expansum, P. verrucosum), Aspergillus (A. kidneys with eventual necrosis (Friss et al., terreus), and Monascus (M. ruber) were 1969; Jordan et al., 1978; Krogh, 1978, also found to produce this mycotoxin Krogh et al., 1974). In mice, citrinin is (Ciegler et al., 1977, Bragulat et al., 1977). also embryocidal and fetotoxic (Hood et CTN contaminates maize (Nelson et al., al., 1976). In rats, citrinin has similar 1985), wheat, rye, barley, oats (Scott et effects and high doses are teratogenic al.,1972), and rice (Tanaka et al., 2007). (Reddy et al., 1982a,b). Citrinin has been Citrinin was considered as a potential implicated in porcine and avian antibiotic (IARC, 1986), but its toxic nephropathies and possibly in the fatal properties prevented its therapeutic use. renal disease in humans, causing endemic Citrinin (CTN), a low molecular weight Balkan nephropathy (Friss et al., 1969;, compound, that melts at 172oC and is Krogh et al., 1970 Witlock, et al., 1977). -

Naturally Occurring Toxins in Foods
101 Naturally occurring toxins in foods By I. D. MORTON,Department of Food Science, Queen Elizabeth College, London W8 7AH Toxic materials in foodstuffs can be present naturally or they can occur due to the changes taking place during harvesting, processing or preservation of food. Much has been written in the past about the simpler materials present in foodstuffs which are toxic. I am thinking here of materials such as phytic acid present in the wheat grain and the presence of oxalic acid in the leaves of the rhubarb plant. Less, however, is known about materials present in many of the legumes which require heat treatment before the legumes or the products from the legumes are safe for human consumption. A modem classification of toxic materials could be as follows: (A) simple compounds present as such in foods; (B) more complicated compounds present in foods which must be and can be removed by treatment before the food is consumed; (C) toxic materials produced by processing and preservation; (D) complicated compounds present in foods which unfortunately are not removed by processing. We are all accustomed to the use of spices with our foodstuffs. Pepper and salt have been used for centuries. It is, perhaps, not quite so well known that the material present in the ordinary nutmeg is related to lysergic acid and that two whole nutmegs ground up would be sufficient for hallucinations in most people. Many of the essential oils are toxic and a number have been shown to be carcino- genic. Some of the flavouring agents are known also to be carcinogenic. -

Occurrence of Penicillium Brocae and Penicillium Citreonigrum, Which
toxins Article Occurrence of Penicillium brocae and Penicillium citreonigrum, which Produce a Mutagenic Metabolite and a Mycotoxin Citreoviridin, Respectively, in Selected Commercially Available Rice Grains in Thailand Nozomi Shiratori 1,†, Naoki Kobayashi 1,2,†, Phitsanu Tulayakul 3, Yoshitsugu Sugiura 1, Masahiko Takino 4, Osamu Endo 2 and Yoshiko Sugita-Konishi 1,2,* 1 Graduate School of Life and Environmental Sciences, Azabu University, 1-17-71, Fuchinobe Chuo-ku, Sagamihara 252-5201, Japan; [email protected] (N.S.); [email protected] (N.K.); [email protected] (Y.S.) 2 Faculty of Life and Environmental Sciences, Azabu University, 1-17-71, Fuchinobe Chuo-ku, Sagamihara 252-5201, Japan; [email protected] 3 Department of Veterinary Public Health, Faculty of Veterinary Medicine, Kasetsart University, Kamphaeng Saen Campus, Nakhon Pathom 73140, Thailand; [email protected] 4 Agilent Technologies, Japan, Ltd., 9-1 Takakura-cho, Hachioji, Tokyo 192-8510, Japan; [email protected] * Correspondence: [email protected]; Tel.: +81-42-754-7111 (ext. 2367) or +81-42-767-1887; Fax: +81-42-767-1887 † These authors contributed equally to this work. Academic Editor: Paola Battilani Received: 17 February 2017; Accepted: 12 June 2017; Published: 15 June 2017 Abstract: Commercially available rice grains in Thailand were examined to isolate the monoverticillate Penicillium species responsible for toxic yellowed rice. Penicillium species were obtained from seven out of 10 rice samples tested. Among them, one Penicillium citreonigrum isolate and six Penicillium brocae isolates were morphologically identified. The P. citreonigrum isolate produced the mycotoxin citreoviridin on a yeast extract sucrose broth medium.