Tau Protein in Lung Smooth Muscle Cells
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guidelines for Scholarships Theta Pi Chapter of Sigma Theta Tau Illinois
Guidelines for Scholarships Theta Pi Chapter of Sigma Theta Tau Illinois Wesleyan University Research/Awards Subcommittee The Research/Awards Subcommittee of the Leadership Succession Committee consists of three appointed chapter members and others as appointed who have experience in nursing education and/or research. The Research/Awards Subcommittee reviews applications, recommends individuals for scholarships, and monitors fund usage by recipients. A five-year record is kept of all recipients of scholarships, including recipients’ names and addresses, amounts of awards, and criteria used for selection of recipients. The Board of Directors approves the recommendations of the Research/Awards Subcommittee based upon availability of funds for scholarships. The treasurer distributes scholarship money to the recipients. Purpose of the Scholarship The purpose of the scholarship is to support outstanding nursing students who have the potential to advance nursing knowledge in the area of nursing science and practice. The fund provides money for one or more scholarships. Eligibility 1. Applicant is enrolled in a baccalaureate program in nursing, a higher degree program in nursing, or a doctoral program. 2. Preference will be given to applicants who are members of Theta Pi Chapter of STTI. Criteria for Selection 1. Quality of written goals 2. Contribution or potential contribution to nursing and public benefit 3. Availability of funds in the scholarship fund budget and number of applications received 4. Grade point average Amount of Award The amount of the award may vary from year to year, depending upon the funds requested, the number of requests, and the money available in the chapter scholarship fund. The scholarship may be used for any legitimate expense associated with academic study specified under eligibility. -

Lung Anatomy
Lung anatomy Breathing Breathing is an automatic and usually subconscious process which is controlled by the brain. The brain will determine how much oxygen we require and how fast we need to breathe in order to supply our vital organs (brain, heart, kidneys, liver, stomach and bowel), as well as our muscles and joints, with enough oxygen to carry out our normal daily activities. In order for breathing to be effective we need to use our lungs, breathing muscles and blood system efficiently. This leaflet should help you to better understand the process of breathing and how we get the much needed oxygen into our bodies. The lungs You have two lungs, one in the right side and one in the left side of your chest. The right lung is bigger than the left due to the position of the heart (which is positioned in the left side of the chest). Source: Pulmonary Rehabilitation Reference No: 66354-1 Issue date: 13/2/20 Review date: 13/2/23 Page 1 of 5 Both lungs are covered by 2 thin layers of tissue called the pleura. The pleura stop the surface of the lungs rubbing together as we breathe in and out. The lungs are protected by the ribcage. The airways Within the lungs there is a vast network of airways (tubes) which help to transport the oxygen into the lungs and the carbon dioxide out. These tubes branch into smaller and smaller tubes the further they go into the lungs. Page 2 of 5 Trachea (windpipe): This tube connects your nose and mouth to your lungs. -

Conserved Microtubule–Actin Interactions in Cell Movement and Morphogenesis
REVIEW Conserved microtubule–actin interactions in cell movement and morphogenesis Olga C. Rodriguez, Andrew W. Schaefer, Craig A. Mandato, Paul Forscher, William M. Bement and Clare M. Waterman-Storer Interactions between microtubules and actin are a basic phenomenon that underlies many fundamental processes in which dynamic cellular asymmetries need to be established and maintained. These are processes as diverse as cell motility, neuronal pathfinding, cellular wound healing, cell division and cortical flow. Microtubules and actin exhibit two mechanistic classes of interactions — regulatory and structural. These interactions comprise at least three conserved ‘mechanochemical activity modules’ that perform similar roles in these diverse cell functions. Over the past 35 years, great progress has been made towards under- crosstalk occurs in processes that require dynamic cellular asymme- standing the roles of the microtubule and actin cytoskeletal filament tries to be established or maintained to allow rapid intracellular reor- systems in mechanical cellular processes such as dynamic shape ganization or changes in shape or direction in response to stimuli. change, shape maintenance and intracellular organelle movement. Furthermore, the widespread occurrence of these interactions under- These functions are attributed to the ability of polarized cytoskeletal scores their importance for life, as they occur in diverse cell types polymers to assemble and disassemble rapidly, and to interact with including epithelia, neurons, fibroblasts, oocytes and early embryos, binding proteins and molecular motors that mediate their regulated and across species from yeast to humans. Thus, defining the mecha- movement and/or assembly into higher order structures, such as radial nisms by which actin and microtubules interact is key to understand- arrays or bundles. -

Absence of NEFL in Patient-Specific Neurons in Early-Onset Charcot-Marie-Tooth Neuropathy Markus T
ARTICLE OPEN ACCESS Absence of NEFL in patient-specific neurons in early-onset Charcot-Marie-Tooth neuropathy Markus T. Sainio, MSc, Emil Ylikallio, MD, PhD, Laura M¨aenp¨a¨a, MSc, Jenni Lahtela, PhD, Pirkko Mattila, PhD, Correspondence Mari Auranen, MD, PhD, Johanna Palmio, MD, PhD, and Henna Tyynismaa, PhD Dr. Tyynismaa [email protected] Neurol Genet 2018;4:e244. doi:10.1212/NXG.0000000000000244 Abstract Objective We used patient-specific neuronal cultures to characterize the molecular genetic mechanism of recessive nonsense mutations in neurofilament light (NEFL) underlying early-onset Charcot- Marie-Tooth (CMT) disease. Methods Motor neurons were differentiated from induced pluripotent stem cells of a patient with early- onset CMT carrying a novel homozygous nonsense mutation in NEFL. Quantitative PCR, protein analytics, immunocytochemistry, electron microscopy, and single-cell transcriptomics were used to investigate patient and control neurons. Results We show that the recessive nonsense mutation causes a nearly total loss of NEFL messenger RNA (mRNA), leading to the complete absence of NEFL protein in patient’s cultured neurons. Yet the cultured neurons were able to differentiate and form neuronal networks and neuro- filaments. Single-neuron gene expression fingerprinting pinpointed NEFL as the most down- regulated gene in the patient neurons and provided data of intermediate filament transcript abundancy and dynamics in cultured neurons. Blocking of nonsense-mediated decay partially rescued the loss of NEFL mRNA. Conclusions The strict neuronal specificity of neurofilament has hindered the mechanistic studies of re- cessive NEFL nonsense mutations. Here, we show that such mutation leads to the absence of NEFL, causing childhood-onset neuropathy through a loss-of-function mechanism. -

Lung Microbiome Participation in Local Immune Response Regulation in Respiratory Diseases
microorganisms Review Lung Microbiome Participation in Local Immune Response Regulation in Respiratory Diseases Juan Alberto Lira-Lucio 1 , Ramcés Falfán-Valencia 1 , Alejandra Ramírez-Venegas 2, Ivette Buendía-Roldán 3 , Jorge Rojas-Serrano 4 , Mayra Mejía 4 and Gloria Pérez-Rubio 1,* 1 HLA Laboratory, Instituto Nacional de Enfermedades Respiratorias Ismael Cosío Villegas, Mexico City 14080, Mexico; [email protected] (J.A.L.-L.); [email protected] (R.F.-V.) 2 Tobacco Smoking and COPD Research Department, Instituto Nacional de Enfermedades Respiratorias Ismael Cosío Villegas, Mexico City 14080, Mexico; [email protected] 3 Translational Research Laboratory on Aging and Pulmonary Fibrosis, Instituto Nacional de Enfermedades Respiratorias Ismael Cosío Villegas, Mexico City 14080, Mexico; [email protected] 4 Interstitial Lung Disease and Rheumatology Unit, Instituto Nacional de Enfermedades Respiratorias Ismael Cosío Villegas, Mexico City 14080, Mexico; [email protected] (J.R.-S.); [email protected] (M.M.) * Correspondence: [email protected]; Tel.: +52-55-5487-1700 (ext. 5152) Received: 11 June 2020; Accepted: 7 July 2020; Published: 16 July 2020 Abstract: The lung microbiome composition has critical implications in the regulation of innate and adaptive immune responses. Next-generation sequencing techniques have revolutionized the understanding of pulmonary physiology and pathology. Currently, it is clear that the lung is not a sterile place; therefore, the investigation of the participation of the pulmonary microbiome in the presentation, severity, and prognosis of multiple pathologies, such as asthma, chronic obstructive pulmonary disease, and interstitial lung diseases, contributes to a better understanding of the pathophysiology. Dysregulation of microbiota components in the microbiome–host interaction is associated with multiple lung pathologies, severity, and prognosis, making microbiome study a useful tool for the identification of potential therapeutic strategies. -

Microtubule-Associated Protein Tau (Molecular Pathology/Neurodegenerative Disease/Neurofibriliary Tangles) M
Proc. Nati. Acad. Sci. USA Vol. 85, pp. 4051-4055, June 1988 Medical Sciences Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease: Identification as the microtubule-associated protein tau (molecular pathology/neurodegenerative disease/neurofibriliary tangles) M. GOEDERT*, C. M. WISCHIK*t, R. A. CROWTHER*, J. E. WALKER*, AND A. KLUG* *Medical Research Council Laboratory of Molecular Biology, Hills Road, Cambridge CB2 2QH, United Kingdom; and tDepartment of Psychiatry, University of Cambridge Clinical School, Hills Road, Cambridge CB2 2QQ, United Kingdom Contributed by A. Klug, March 1, 1988 ABSTRACT Screening of cDNA libraries prepared from (21). This task is made all the more difficult because there is the frontal cortex ofan zheimer disease patient and from fetal no functional or physiological assay for the protein(s) of the human brain has led to isolation of the cDNA for a core protein PHF. The only identification so far possible is the morphol- of the paired helical fiament of Alzheimer disease. The partial ogy of the PHFs at the electron microscope level, and here amino acid sequence of this core protein was used to design we would accept only experiments on isolated individual synthetic oligonucleotide probes. The cDNA encodes a protein of filaments, not on neurofibrillary tangles (in which other 352 amino acids that contains a characteristic amino acid repeat material might be occluded). One thus needs a label or marker in its carboxyl-terminal half. This protein is highly homologous for the PHF itself, which can at the same time be used to to the sequence ofthe mouse microtubule-assoiated protein tau follow the steps of the biochemical purification. -

The Selnolig Package: Selective Suppression of Typographic Ligatures*
The selnolig package: Selective suppression of typographic ligatures* Mico Loretan† 2015/10/26 Abstract The selnolig package suppresses typographic ligatures selectively, i.e., based on predefined search patterns. The search patterns focus on ligatures deemed inappropriate because they span morpheme boundaries. For example, the word shelfful, which is mentioned in the TEXbook as a word for which the ff ligature might be inappropriate, is automatically typeset as shelfful rather than as shelfful. For English and German language documents, the selnolig package provides extensive rules for the selective suppression of so-called “common” ligatures. These comprise the ff, fi, fl, ffi, and ffl ligatures as well as the ft and fft ligatures. Other f-ligatures, such as fb, fh, fj and fk, are suppressed globally, while making exceptions for names and words of non-English/German origin, such as Kafka and fjord. For English language documents, the package further provides ligature suppression rules for a number of so-called “discretionary” or “rare” ligatures, such as ct, st, and sp. The selnolig package requires use of the LuaLATEX format provided by a recent TEX distribution, e.g., TEXLive 2013 and MiKTEX 2.9. Contents 1 Introduction ........................................... 1 2 I’m in a hurry! How do I start using this package? . 3 2.1 How do I load the selnolig package? . 3 2.2 Any hints on how to get started with LuaLATEX?...................... 4 2.3 Anything else I need to do or know? . 5 3 The selnolig package’s approach to breaking up ligatures . 6 3.1 Free, derivational, and inflectional morphemes . -

Neurofilaments: Neurobiological Foundations for Biomarker Applications
Neurofilaments: neurobiological foundations for biomarker applications Arie R. Gafson1, Nicolas R. Barthelmy2*, Pascale Bomont3*, Roxana O. Carare4*, Heather D. Durham5*, Jean-Pierre Julien6,7*, Jens Kuhle8*, David Leppert8*, Ralph A. Nixon9,10,11,12*, Roy Weller4*, Henrik Zetterberg13,14,15,16*, Paul M. Matthews1,17 1 Department of Brain Sciences, Imperial College, London, UK 2 Department of Neurology, Washington University School of Medicine, St Louis, MO, USA 3 a ATIP-Avenir team, INM, INSERM , Montpellier university , Montpellier , France. 4 Clinical Neurosciences, Faculty of Medicine, University of Southampton, Southampton General Hospital, Southampton, United Kingdom 5 Department of Neurology and Neurosurgery, Montreal Neurological Institute, McGill University, Montreal, Québec, Canada 6 Department of Psychiatry and Neuroscience, Laval University, Quebec, Canada. 7 CERVO Brain Research Center, 2601 Chemin de la Canardière, Québec, QC, G1J 2G3, Canada 8 Neurologic Clinic and Policlinic, Departments of Medicine, Biomedicine and Clinical Research, University Hospital Basel, University of Basel, Basel, Switzerland. 9 Center for Dementia Research, Nathan Kline Institute, Orangeburg, NY, 10962, USA. 10Departments of Psychiatry, New York University School of Medicine, New York, NY, 10016, 11 Neuroscience Institute, New York University School of Medicine, New York, NY, 10016, USA. 12Department of Cell Biology, New York University School of Medicine, New York, NY, 10016, USA 13 University College London Queen Square Institute of Neurology, London, UK 14 UK Dementia Research Institute at University College London 15 Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, the Sahlgrenska Academy at the University of Gothenburg, Mölndal, Sweden 16 Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, Mölndal, Sweden 17 UK Dementia Research Institute at Imperial College, London * Co-authors ordered alphabetically Address for correspondence: Prof. -

Manatee Anatomy and Physiology
Manatee Anatomy and Physiology Grade level: Elementary 5 Subject Area: Biology, Anatomy and Physiology, Marine Biology Duration: Teach: 15 minutes, Activity: 20 minutes, Discussion: 20 minutes. Setting: Classroom Next Generation Sunshine State Standards: Reading: LAFS.5.RI Reading standards for Information text Cluster 3 LAFS.5.RI.3 Writing: LA.B.2.1, LA.B.1.2 Speaking and Listening: Cluster 2 LAFS.5.SL.Z Presentation of Knowledge and Ideas Science: Body of Knowledge Life Science: SC.5.L.14, SC.5.L.15, SC.5.L.17, Body of Knowledge Nature of Science Supporting Idea 1: SC.5.N.1 Practice of Science, Supporting Idea 2:SC.5.N.2 Characteristics of Scientific Knowledge Earth Systems and Patterns: 7SC.E.E.7 Statewide Science Assessment Prompt: How might humans help manatees survive? Objectives: Students will learn about manatee bodies and explain some anatomical and physiological differences between manatees, humans and other animals. Materials: Handouts of basic manatee anatomy, dolphin anatomy & human anatomy, crayons or markers, coloring direction sheet, question worksheet, Quiz sheet Vocabulary: Mammal, endangered species, habitat, conservation, vibrissae, nares, blowhole, flipper, herbivore, omnivore, carnivore. Background/Preparation: Handouts of manatee, dolphin, and human anatomy. Fact sheets comparing and contrasting specific and unique anatomical aspects of each species. Basic manatee fact sheet highlighting personality, limited habitat, endangered status and conservation efforts. Teachers can review the manatee fact sheets, and select points of interest they would most like to incorporate into a lesson. This activity may fit best into the week where the human anatomy lessons are addressed. Teachers can present the information via traditional lecture, group discussion, question and answer session, or doing the coloring activity as the lesson points are addressed, etc. -

The Fifth Pulmonary Vein
CASE REPORT Anatomy Journal of Africa. 2016. Vol 5 (2): 704 - 706 The Fifth Pulmonary vein Hilkiah Kinfemichael, Asrat Dawit Correspondence to Hilkiah Kinfemichael, Myungsung Medical College, Addis Ababa, Ethiopia PO Box 14972 Email : [email protected]. ABSTRACT A cadaver in Myungsung Medical College (MMC) had a 3rd pulmonary vein originating from the middle lobe of the right lung. Such anatomical variations are very rare. People with this variation have a total of five pulmonary veins entering left atrium. It has clinical implications especially for thoracic surgeons and radiologists during radiofrequency ablations, lobectomies, valve replacements, pulmonary vein catheterizations, video-assisted thoracic surgery (VATS) and others. Key Words: Anatomy, Variations, Pulmonary veins. INTRODUCTION Two pulmonary veins usually drain oxygenated upper lobe, is formed by the union of blood from each lung to left atrium of the apicoposterior, anterior and lingular veins. The heart. The lobular tributaries lie mainly in the inferior left pulmonary vein, which drains the interlobular septa and two pulmonary veins lower lobe, is formed by the hilar union of two from each lung enter left atrium through two veins, superior and common basal veins. The separate pulmonary ostia on either side. On the right and left pulmonary veins perforate the right lung veins from the apical, anterior and fibrous pericardium and open separately in the posterior part of upper lobe unite with a middle posterosuperior aspect of the left atrium lobar vein, which is formed by lateral and (Standring et al., 2008). This anatomy could medial tributaries, and form the superior right show variations, with more veins draining pulmonary vein (Standring, 2008). -

Expression of Separate Isoforms of Human Tau Protein: Correlation with the Tau Pattern in Brain and Effects on Tubulin Polymerization
The EMBO Journal vol.9 no.13 pp.4225-4230, 1990 Expression of separate isoforms of human tau protein: correlation with the tau pattern in brain and effects on tubulin polymerization Michel Goedert and Ross Jakes half of the molecule. Experiments using tau fragments synthesized in vitro and synthetic peptides have shown that Medical Research Council Laboratory of Molecular Biology, Hills the repeats constitute microtubule binding units (Aizawa et Road, Cambridge CB2 2QH, UK al., 1989; Ennulat et al., 1989; Himmler et al., 1989; Lee Communicated by R.A.Crowther et al., 1989). Additional isoforms exist which contain 29 or 58 amino acid insertions in the amino-terminal region in conjunction with three or four repeats, giving rise in humans We have expressed six previously cloned isoforms of to a total of six different isoforms identified from full-length human microtubule-associated tau protein in Escherichia cDNA clones (Goedert et al., 1989b). The shortest human coli and purified them to homogeneity in a biologically form is 352 amino acids in length and contains three repeats, active form. They range from 352 to 441 amino acids in whereas the largest form is 441 amino acids in length and length and differ from each other by the presence of three contains four repeats and the 58 amino acid insertion. By or four tandem repeats in the carboxy-terminal half and RNase protection assay on human brain, mRNAs encoding by the presence or absence of 29 or 58 amino acid inserts three-repeat containing isoforms are found both in fetal and in the amino-terminus. -
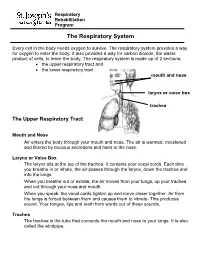
The Respiratory System
Respiratory Rehabilitation Program The Respiratory System Every cell in the body needs oxygen to survive. The respiratory system provides a way for oxygen to enter the body. It also provides a way for carbon dioxide, the waste product of cells, to leave the body. The respiratory system is made up of 2 sections: the upper respiratory tract and the lower respiratory tract mouth and nose larynx or voice box trachea The Upper Respiratory Tract Mouth and Nose Air enters the body through your mouth and nose. The air is warmed, moistened and filtered by mucous secretions and hairs in the nose. Larynx or Voice Box The larynx sits at the top of the trachea. It contains your vocal cords. Each time you breathe in or inhale, the air passes through the larynx, down the trachea and into the lungs. When you breathe out or exhale, the air moves from your lungs, up your trachea and out through your nose and mouth. When you speak, the vocal cords tighten up and move closer together. Air from the lungs is forced between them and causes them to vibrate. This produces sound. Your tongue, lips and teeth form words out of these sounds. Trachea The trachea is the tube that connects the mouth and nose to your lungs. It is also called the windpipe. The Lower Respiratory Tract Inside Lungs Outside Lungs bronchial tubes alveoli diaphragm (muscle) Bronchial Tubes The trachea splits into 2 bronchial tubes in your lungs. These are called the left bronchus and right bronchus. The bronchus tubes keep branching off into smaller and smaller tubes called bronchi.