Dichotomous Key to Orders
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ecomorph Convergence in Stick Insects (Phasmatodea) with Emphasis on the Lonchodinae of Papua New Guinea
Brigham Young University BYU ScholarsArchive Theses and Dissertations 2018-07-01 Ecomorph Convergence in Stick Insects (Phasmatodea) with Emphasis on the Lonchodinae of Papua New Guinea Yelena Marlese Pacheco Brigham Young University Follow this and additional works at: https://scholarsarchive.byu.edu/etd Part of the Life Sciences Commons BYU ScholarsArchive Citation Pacheco, Yelena Marlese, "Ecomorph Convergence in Stick Insects (Phasmatodea) with Emphasis on the Lonchodinae of Papua New Guinea" (2018). Theses and Dissertations. 7444. https://scholarsarchive.byu.edu/etd/7444 This Thesis is brought to you for free and open access by BYU ScholarsArchive. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Ecomorph Convergence in Stick Insects (Phasmatodea) with Emphasis on the Lonchodinae of Papua New Guinea Yelena Marlese Pacheco A thesis submitted to the faculty of Brigham Young University in partial fulfillment of the requirements for the degree of Master of Science Michael F. Whiting, Chair Sven Bradler Seth M. Bybee Steven D. Leavitt Department of Biology Brigham Young University Copyright © 2018 Yelena Marlese Pacheco All Rights Reserved ABSTRACT Ecomorph Convergence in Stick Insects (Phasmatodea) with Emphasis on the Lonchodinae of Papua New Guinea Yelena Marlese Pacheco Department of Biology, BYU Master of Science Phasmatodea exhibit a variety of cryptic ecomorphs associated with various microhabitats. Multiple ecomorphs are present in the stick insect fauna from Papua New Guinea, including the tree lobster, spiny, and long slender forms. While ecomorphs have long been recognized in phasmids, there has yet to be an attempt to objectively define and study the evolution of these ecomorphs. -

Nuisance Insects and Climate Change
www.defra.gov.uk Nuisance Insects and Climate Change March 2009 Department for Environment, Food and Rural Affairs Nobel House 17 Smith Square London SW1P 3JR Tel: 020 7238 6000 Website: www.defra.gov.uk © Queen's Printer and Controller of HMSO 2007 This publication is value added. If you wish to re-use this material, please apply for a Click-Use Licence for value added material at http://www.opsi.gov.uk/click-use/value-added-licence- information/index.htm. Alternatively applications can be sent to Office of Public Sector Information, Information Policy Team, St Clements House, 2-16 Colegate, Norwich NR3 1BQ; Fax: +44 (0)1603 723000; email: [email protected] Information about this publication and further copies are available from: Local Environment Protection Defra Nobel House Area 2A 17 Smith Square London SW1P 3JR Email: [email protected] This document is also available on the Defra website and has been prepared by Centre of Ecology and Hydrology. Published by the Department for Environment, Food and Rural Affairs 2 An Investigation into the Potential for New and Existing Species of Insect with the Potential to Cause Statutory Nuisance to Occur in the UK as a Result of Current and Predicted Climate Change Roy, H.E.1, Beckmann, B.C.1, Comont, R.F.1, Hails, R.S.1, Harrington, R.2, Medlock, J.3, Purse, B.1, Shortall, C.R.2 1Centre for Ecology and Hydrology, 2Rothamsted Research, 3Health Protection Agency March 2009 3 Contents Summary 5 1.0 Background 6 1.1 Consortium to perform the work 7 1.2 Objectives 7 2.0 -

A Remarkable Caddisfly with Bipectinate Antennae in Cretaceous
Cretaceous Research 69 (2017) 198e203 Contents lists available at ScienceDirect Cretaceous Research journal homepage: www.elsevier.com/locate/CretRes Short communication A remarkable caddisfly with bipectinate antennae in Cretaceous Burmese amber (Insecta, Trichoptera) * Wilfried Wichard a, Bo Wang b, c, a Institute of Biology, University of Koeln, Gronewaldstr. 2, D 50931 Koeln, Germany b State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, Nanjing 210008, China c Key Laboratory of Zoological Systematics and Evolution, Institute of Zoology, Chinese Academy of Science, Beijing 100101, China article info abstract Article history: A new caddisfly (Trichoptera), Palaeopsilotreta xiai gen. et sp. nov. is described based on three well- Received 8 August 2016 preserved male specimens from mid-Cretaceous Burmese amber. It is assigned to the extant family Received in revised form Odontoceridae. Palaeopsilotreta is similar to the extant genus Psilotreta but differs from the latter by 19 September 2016 partially bipectinate antennae which are unknown among living Trichoptera. Our fossils are not only the Accepted in revised form 28 September only Mesozoic Odontoceridae, but also hitherto the earliest record of this family. 2016 © Available online 29 September 2016 2016 Elsevier Ltd. All rights reserved. Keywords: Taxonomy Fossil caddisfly Palaeopsilotreta xiai Psilotreta Odontoceridae 1. Introduction insights into the evolution of this lineage. In this paper, we describe a new extinct genus and species placed in the family Odontocer- Burmese amber (from northern Myanmar) contains the most idae: Palaeopsilotreta xiai gen. et sp. nov., based on three well- diverse biota in amber from the mid-Cretaceous and more than 250 preserved male specimens. -

Minutes of the January 25, 2010, Meeting of the Board of Regents
MINUTES OF THE JANUARY 25, 2010, MEETING OF THE BOARD OF REGENTS ATTENDANCE This scheduled meeting of the Board of Regents was held on Monday, January 25, 2010, in the Regents’ Room of the Smithsonian Institution Castle. The meeting included morning, afternoon, and executive sessions. Board Chair Patricia Q. Stonesifer called the meeting to order at 8:31 a.m. Also present were: The Chief Justice 1 Sam Johnson 4 John W. McCarter Jr. Christopher J. Dodd Shirley Ann Jackson David M. Rubenstein France Córdova 2 Robert P. Kogod Roger W. Sant Phillip Frost 3 Doris Matsui Alan G. Spoon 1 Paul Neely, Smithsonian National Board Chair David Silfen, Regents’ Investment Committee Chair 2 Vice President Joseph R. Biden, Senators Thad Cochran and Patrick J. Leahy, and Representative Xavier Becerra were unable to attend the meeting. Also present were: G. Wayne Clough, Secretary John Yahner, Speechwriter to the Secretary Patricia L. Bartlett, Chief of Staff to the Jeffrey P. Minear, Counselor to the Chief Justice Secretary T.A. Hawks, Assistant to Senator Cochran Amy Chen, Chief Investment Officer Colin McGinnis, Assistant to Senator Dodd Virginia B. Clark, Director of External Affairs Kevin McDonald, Assistant to Senator Leahy Barbara Feininger, Senior Writer‐Editor for the Melody Gonzales, Assistant to Congressman Office of the Regents Becerra Grace L. Jaeger, Program Officer for the Office David Heil, Assistant to Congressman Johnson of the Regents Julie Eddy, Assistant to Congresswoman Matsui Richard Kurin, Under Secretary for History, Francisco Dallmeier, Head of the National Art, and Culture Zoological Park’s Center for Conservation John K. -

Insect Orders V: Panorpida & Hymenoptera
Insect Orders V: Panorpida & Hymenoptera • The Panorpida contain 5 orders: the Mecoptera, Siphonaptera, Diptera, Trichoptera and Lepidoptera. • Available evidence clearly indicates that the Lepidoptera and the Trichoptera are sister groups. • The Siphonaptera and Mecoptera are also closely related but it is not clear whether the Siponaptera is the sister group of all of the Mecoptera or a group (Boreidae) within the Mecoptera. If the latter is true, then the Mecoptera is paraphyletic as currently defined. • The Diptera is the sister group of the Siphonaptera + Mecoptera and together make up the Mecopteroids. • The Hymenoptera does not appear to be closely related to any of the other holometabolous orders. Mecoptera (Scorpionflies, hangingflies) • Classification. 600 species worldwide, arranged into 9 families (5 in the US). A very old group, many fossils from the Permian (260 mya) onward. • Structure. Most distinctive feature is the elongated clypeus and labrum that together form a rostrum. The order gets its common name from the gential segment of the male in the family Panorpodiae, which is bulbous and often curved forward above the abdomen, like the sting of a scorpion. Larvae are caterpillar-like or grub- like. • Natural history. Scorpionflies are most common in cool, moist habitats. They get the name “hangingflies” from their habit of hanging upside down on vegetation. Larvae and adult males are mostly predators or scavengers. Adult females are usually scavengers. Larvae and adults in some groups may feed on vegetation. Larvae of most species are terrestrial and caterpillar-like in body form. Larvae of some species are aquatic. In the family Bittacidae males attract females for mating by releasing a sex pheromone and then presenting the female with a nuptial gift. -

Insecta: Phasmatodea) and Their Phylogeny
insects Article Three Complete Mitochondrial Genomes of Orestes guangxiensis, Peruphasma schultei, and Phryganistria guangxiensis (Insecta: Phasmatodea) and Their Phylogeny Ke-Ke Xu 1, Qing-Ping Chen 1, Sam Pedro Galilee Ayivi 1 , Jia-Yin Guan 1, Kenneth B. Storey 2, Dan-Na Yu 1,3 and Jia-Yong Zhang 1,3,* 1 College of Chemistry and Life Science, Zhejiang Normal University, Jinhua 321004, China; [email protected] (K.-K.X.); [email protected] (Q.-P.C.); [email protected] (S.P.G.A.); [email protected] (J.-Y.G.); [email protected] (D.-N.Y.) 2 Department of Biology, Carleton University, Ottawa, ON K1S 5B6, Canada; [email protected] 3 Key Lab of Wildlife Biotechnology, Conservation and Utilization of Zhejiang Province, Zhejiang Normal University, Jinhua 321004, China * Correspondence: [email protected] or [email protected] Simple Summary: Twenty-seven complete mitochondrial genomes of Phasmatodea have been published in the NCBI. To shed light on the intra-ordinal and inter-ordinal relationships among Phas- matodea, more mitochondrial genomes of stick insects are used to explore mitogenome structures and clarify the disputes regarding the phylogenetic relationships among Phasmatodea. We sequence and annotate the first acquired complete mitochondrial genome from the family Pseudophasmati- dae (Peruphasma schultei), the first reported mitochondrial genome from the genus Phryganistria Citation: Xu, K.-K.; Chen, Q.-P.; Ayivi, of Phasmatidae (P. guangxiensis), and the complete mitochondrial genome of Orestes guangxiensis S.P.G.; Guan, J.-Y.; Storey, K.B.; Yu, belonging to the family Heteropterygidae. We analyze the gene composition and the structure D.-N.; Zhang, J.-Y. -

Insect Orders
CMG GardenNotes #313 Insect Orders Outline Anoplura: sucking lice, page 1 Blattaria: cockroaches and woodroaches, page 2 Coleoptera: beetles, page 2 Collembola: springtails, page 4 Dermaptera: earwigs, page 4 Diptera: flies, page 5 Ephemeroptera: mayflies, page 6 Hemiptera (suborder Heteroptera): true bugs, page 7 Hemiptera (suborders Auchenorrhyncha and Sternorrhyncha): aphids, cicadas, leafhoppers, mealybugs, scale and whiteflies, page 8 Hymenoptera: ants, bees, horntails, sawflies, and wasp, page 9 Isoptera: termites, page 11 Lepidoptera: butterflies and moths, page 12 Mallophaga: chewing and biting lice, page 13 Mantodea: mantids, page 14 Neuroptera: antlions, lacewings, snakeflies and dobsonflies, page 14 Odonata: dragonflies and damselflies, page 15 Orthoptera: crickets, grasshoppers, and katydids, page 15 Phasmida: Walking sticks, page 16 Plecoptera: stoneflies, page 16 Psocoptera: Psocids or booklice, page 17 Siphonaptera: Fleas, page 17 Thysanoptera: Thrips, page 17 Trichoptera: Caddisflies, page 18 Zygentomaa: Silverfish and Firebrats, page 18 Anoplura Sucking Lice • Feeds by sucking blood from mammals. • Some species (head lice and crabs lice) feed on humans. Metamorphosis: Simple/Gradual Features: [Figure 1] Figure 1. Sucking lice o Wingless o Mouthparts: Piercing/sucking, designed to feed on blood. o Body: Small head with larger, pear-shaped thorax and nine segmented abdomen. 313-1 Blattaria (Subclass of Dictyoptera) Cockroaches and Woodroaches • Most species are found in warmer subtropical to tropical climates. • The German, Oriental and American cockroach are indoor pests. • Woodroaches live outdoors feeding on decaying bark and other debris. Metamorphosis: Simple/Gradual Figure 2. American cockroach Features: [Figure 2] o Body: Flattened o Antennae: Long, thread-like o Mouthparts: Chewing o Wings: If present, are thickened, semi-transparent with distinct veins and lay flat. -

Silverfish and Firebrats
SilverfiSh and firebratS Integrated Pest Management In and Around the Home If items on your bookshelf have Although small nymphs (those that are chewed-on pages and bindings, sus- less than 1/8 inch long) lack scales, both pect the look-alike household pests large nymphs and adults have them. If silverfish and firebrats. Both insects you see scales around or beneath dam- have enzymes in their gut that digest aged items, it is a good indication that cellulose, and they choose book cases, these pests are the culprits. The scales closets, and places where books, cloth- are delicate, dustlike, and slightly in- ing, starch, or dry foods are available. candescent in the light, and they stick to most surfaces. Silverfish and firebrats are nocturnal and hide during the day. If the object LIFE CYCLE Figure 1. Adult firebrat (left) and silver- they are hiding beneath is moved, they fish. Eggs of both species are about 1/25 of will dart toward another secluded an inch long. The females lay the eggs place. They come out at night to seek in crevices, on cloth, or buried in food food and water. Both insects prefer or dust. The average clutch contains 50 dry food such as cereals, flour, pasta, eggs, but this can vary from 1 to 200. and pet food; paper with glue or paste; Firebrat eggs hatch in about 14 days and sizing in paper including wall paper; silverfish eggs in about 19 to 32 days. In book bindings; and starch in cloth- colder environments eggs can remain ing. -

Biodiversa-Project Description-Final Version-110213
1.A. Detailed description of the research area and research plan Context of the proposal Biological invasions (bioinvasions) are defined as the successful establishment and spread of species outside their native range. They act as a major driver of global changes in species distribution. Diverse organisms and ecosystems may be involved, and although not all invasions have a negative impact, the ecological consequences often include the loss of native biological diversity and changes in community structure and ecosystem activity. There may also be additional negative effects on agriculture, forests, fisheries, and human health. National governments, intergovernmental structures like the European Commission and international organizations such as EPPO, CABI and IUCN have therefore mobilized to (i) introduce international laws on invasive species, (ii) organize international networks of scientists and stakeholders to study bioinvasions, and (iii) formalize the cooperation between national environmental or agricultural protection agencies (e.g. the French Agence Nationale de Sécurité Sanitaire, ANSES). Several billion euros are spent annually to address the problems caused by bioinvasions and the scientific community has focused on predicting and controlling future invasions by understanding how they occur. A peer-reviewed journal entitled "Biological Invasions” has been published since 1999. Ecologists have long drawn attention to the negative ecological effects of invasive species, whereas the evolutionary aspects of bioinvasions have received comparatively little attention. This reflects the fact that: i) invasive populations were thought to experience significant bottlenecks during their introduction to new environments and thus possess a limited potential to evolve; and ii) evolution was considered too slow to play a significant role given the relatively short timescale of the invasion process. -

Aquatic Critters Aquatic Critters (Pictures Not to Scale) (Pictures Not to Scale)
Aquatic Critters Aquatic Critters (pictures not to scale) (pictures not to scale) dragonfly naiad↑ ↑ mayfly adult dragonfly adult↓ whirligig beetle larva (fairly common look ↑ water scavenger for beetle larvae) ↑ predaceous diving beetle mayfly naiad No apparent gills ↑ whirligig beetle adult beetle - short, clubbed antenna - 3 “tails” (breathes thru butt) - looks like it has 4 - thread-like antennae - surface head first - abdominal gills Lower jaw to grab prey eyes! (see above) longer than the head - swim by moving hind - surface for air with legs alternately tip of abdomen first water penny -row bklback legs (fbll(type of beetle larva together found under rocks damselfly naiad ↑ in streams - 3 leaf’-like posterior gills - lower jaw to grab prey damselfly adult↓ ←larva ↑adult backswimmer (& head) ↑ giant water bug↑ (toe dobsonfly - swims on back biter) female glues eggs water boatman↑(&head) - pointy, longer beak to back of male - swims on front -predator - rounded, smaller beak stonefly ↑naiad & adult ↑ -herbivore - 2 “tails” - thoracic gills ↑mosquito larva (wiggler) water - find in streams strider ↑mosquito pupa mosquito adult caddisfly adult ↑ & ↑midge larva (males with feather antennae) larva (bloodworm) ↑ hydra ↓ 4 small crustaceans ↓ crane fly ←larva phantom midge larva ↑ adult→ - translucent with silvery bflbuoyancy floats ↑ daphnia ↑ ostracod ↑ scud (amphipod) (water flea) ↑ copepod (seed shrimp) References: Aquatic Entomology by W. Patrick McCafferty ↑ rotifer prepared by Gwen Heistand for ACR Education midge adult ↑ Guide to Microlife by Kenneth G. Rainis and Bruce J. Russel 28 How do Aquatic Critters Get Their Air? Creeks are a lotic (flowing) systems as opposed to lentic (standing, i.e, pond) system. Look for … BREATHING IN AN AQUATIC ENVIRONMENT 1. -
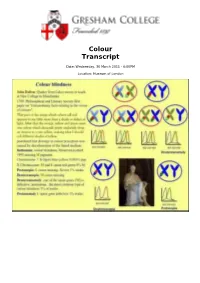
Colour Transcript
Colour Transcript Date: Wednesday, 30 March 2011 - 6:00PM Location: Museum of London 30 March 2011 Colour Professor William Ayliffe Some of you in this audience will be aware that it is the 150th anniversary of the first colour photograph, which was projected at a lecture at the Royal Institute by James Clerk Maxwell. This is the photograph, showing a tartan ribbon, which was taken using the first SLR, invented by Maxwell’s friend. He took three pictures, using three different filters, and was then able to project this gorgeous image, showing three different colours for the first time ever. Colour and Colour Vision This lecture is concerned with the questions: “What is colour?” and “What is colour vision?” - not necessarily the same things. We are going to look at train crashes and colour blindness (which is quite gruesome); the antique use of colour in pigments – ancient red Welsh “Ladies”; the meaning of colour in medieval Europe; discovery of new pigments; talking about colour; language and colour; colour systems and the psychology of colour. So there is a fair amount of ground to cover here, which is appropriate because colour is probably one of the most complex issues that we deal with. The main purpose of this lecture is to give an overview of the whole field of colour, without going into depth with any aspects in particular. Obviously, colour is a function of light because, without light, we cannot see colour. Light is that part of the electromagnetic spectrum that we can see, and that forms only a tiny portion. -

HOST PLANTS of SOME STERNORRHYNCHA (Phytophthires) in NETHERLANDS NEW GUINEA (Homoptera)
Pacific Insects 4 (1) : 119-120 January 31, 1962 HOST PLANTS OF SOME STERNORRHYNCHA (Phytophthires) IN NETHERLANDS NEW GUINEA (Homoptera) By R. T. Simon Thomas DEPARTMENT OF ECONOMIC AFFAIRS, HOLLANDIA In this paper, I list 15 hostplants of some Phytophthires of Netherlands New Guinea. Families, genera within the families and species within the genera are mentioned in alpha betical order. The genera and the specific names of the insects are printed in bold-face type, those of the plants are in italics. The locality, where the insects were found, is printed after the host plants. Then follows the date of collection and finally the name of the collector1 in parentheses. I want to acknowledge my great appreciation for the identification of the Aphididae to Mr. D. Hille Ris Lambers and of the Coccoidea to Dr. A. Reyne. Aphididae Cerataphis variabilis Hrl. Cocos nucifera Linn.: Koor, near Sorong, 26-VII-1961 (S. Th.). Longiunguis sacchari Zehntner. Andropogon sorghum Brot.: Kota Nica2 13-V-1959 (S. Th.). Toxoptera aurantii Fonsc. Citrus sp.: Kota Nica, 16-VI-1961 (S. Th.). Theobroma cacao Linn.: Kota Nica, 19-VIII-1959 (S. Th.), Amban-South, near Manokwari, 1-XII- 1960 (J. Schreurs). Toxoptera citricida Kirkaldy. Citrus sp.: Kota Nica, 16-VI-1961 (S. Th.). Schizaphis cyperi v. d. Goot, subsp, hollandiae Hille Ris Lambers (in litt.). Polytrias amaura O. K.: Hollandia, 22-V-1958 (van Leeuwen). COCCOIDEA Aleurodidae Aleurocanthus sp. Citrus sp.: Kota Nica, 16-VI-1961 (S. Th.). Asterolecaniidae Asterolecanium pustulans (Cockerell). Leucaena glauca Bth.: Kota Nica, 8-X-1960 (S. Th.). 1. My name, as collector, is mentioned thus: "S.