The Ecology of Mountain Quail in Oregon
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CATALINA CALIFORNIA QUAIL (Callipepla Californica Catalinensis) Paul W
II SPECIES ACCOUNTS Andy Birch PDF of Catalina California Quail account from: Shuford, W. D., and Gardali, T., editors. 2008. California Bird Species of Special Concern: A ranked assessment of species, subspecies, and distinct populations of birds of immediate conservation concern in California. Studies of Western Birds 1. Western Field Ornithologists, Camarillo, California, and California Department of Fish and Game, Sacramento. California Bird Species of Special Concern CATALINA CALIFORNIA QUAIL (Callipepla californica catalinensis) Paul W. Collins Criteria Scores Population Trend 0 Santa Range Trend 0 Barbara County Population Size 7.5 Range Size 10 Ventura Endemism 10 County Population Concentration 10 Threats 0 Los San Miguel Is. Santa Cruz Is. Angeles County Anacapa Is. Santa Rosa Is. Santa Barbara Is. Santa Catalina Is. San Nicolas Is. San Clemente Is. Current Year-round Range Historic Year-round Range County Boundaries Kilometers 20 10 0 20 Current and historic (ca. 1944) year-round range of the Catalina California Quail. Birds from Santa Catalina Island (perhaps brought by Native Americans) later introduced successfully to Santa Rosa (1935–1940) and Santa Cruz (late 1940s) islands, but unsuccessfully to San Nicolas Island (1962); quail from mainland populations of C. c. californica introduced unsuccessfully to Santa Cruz (prior to 1875) and San Clemente (late 19th century, 1913) islands. Catalina California Quail Studies of Western Birds 1:107–111, 2008 107 Studies of Western Birds No. 1 SPECIAL CONCERN PRIORITY HISTORIC RANGE AND ABUNDANCE Currently considered a Bird Species of Special IN CALIFORNIA Concern (year round), priority 3. This subspecies Grinnell and Miller (1944) described the Catalina was not included on prior special concern lists California Quail as a “common to abundant” (Remsen 1978, CDFG 1992). -

Body Temperatures in California and Gambel's Quail
150 Baa•oz,o•awand DawsoN, California and Gambel's Quail [1VoL Auk 75 BODY TEMPERATURES IN CALIFORNIA AND GAMBEL'S QUAIL BY GEORGE A. BARTHOLOMEW AND WILLIAM R. DAWSON Tm• paucity of data on the body temperaturesof small gallinaceous birds prompts us to report miscellaneousobservations which we have made over the past severalyears on the body temperaturesof the Cali- fornia Quail, Lophortyxcalifornicus, a commonresident in grasslandand chaparral from southernOregon to southern Baja Cal/fornia, and the Gambel's Quail, L. œambelii,a residentin the desert regionsof south- western United States and northwestern Mexico. The habitats of both speciesare characterizedby high summer temperaturesand moderate winter conditions. We have therefore paid particular attention to capacity for temperature regulation in hot environments. We found that when ambient (environmental)temperature rose, the birds' body temperaturecould rise by as much as 4ø C. (7.2ø F.), without ill effects. Ability to maintain above normal body temperatures higher than ambient temperaturesfacilitates transfer of excessbody heat to the environment. It is probablya major avian adaptationto hot conditions. The data presentedwere acquiredincidental to work on other species and for that reasonare not comprehensive,but they serveto characterize aspectsof temperatureregulation in quail. MATERIALS AND METHODS The observationswere made on 8 adult and 2 juvenile California Quailcaptured on the LosAngeles campus of the Universityof California, and on 5 adult and 2 juvenile Gambers Quail capturedin the Colorado Desert, three miles south of Calipatria, Imperial County, California. The captive animals of both specieswere housedin cagesof half-inch wire mesh wh/ch measured 12 x 12 x 24 inches and had sand-covered floors. -

The Coastal Scrub and Chaparral Bird Conservation Plan
The Coastal Scrub and Chaparral Bird Conservation Plan A Strategy for Protecting and Managing Coastal Scrub and Chaparral Habitats and Associated Birds in California A Project of California Partners in Flight and PRBO Conservation Science The Coastal Scrub and Chaparral Bird Conservation Plan A Strategy for Protecting and Managing Coastal Scrub and Chaparral Habitats and Associated Birds in California Version 2.0 2004 Conservation Plan Authors Grant Ballard, PRBO Conservation Science Mary K. Chase, PRBO Conservation Science Tom Gardali, PRBO Conservation Science Geoffrey R. Geupel, PRBO Conservation Science Tonya Haff, PRBO Conservation Science (Currently at Museum of Natural History Collections, Environmental Studies Dept., University of CA) Aaron Holmes, PRBO Conservation Science Diana Humple, PRBO Conservation Science John C. Lovio, Naval Facilities Engineering Command, U.S. Navy (Currently at TAIC, San Diego) Mike Lynes, PRBO Conservation Science (Currently at Hastings University) Sandy Scoggin, PRBO Conservation Science (Currently at San Francisco Bay Joint Venture) Christopher Solek, Cal Poly Ponoma (Currently at UC Berkeley) Diana Stralberg, PRBO Conservation Science Species Account Authors Completed Accounts Mountain Quail - Kirsten Winter, Cleveland National Forest. Greater Roadrunner - Pete Famolaro, Sweetwater Authority Water District. Coastal Cactus Wren - Laszlo Szijj and Chris Solek, Cal Poly Pomona. Wrentit - Geoff Geupel, Grant Ballard, and Mary K. Chase, PRBO Conservation Science. Gray Vireo - Kirsten Winter, Cleveland National Forest. Black-chinned Sparrow - Kirsten Winter, Cleveland National Forest. Costa's Hummingbird (coastal) - Kirsten Winter, Cleveland National Forest. Sage Sparrow - Barbara A. Carlson, UC-Riverside Reserve System, and Mary K. Chase. California Gnatcatcher - Patrick Mock, URS Consultants (San Diego). Accounts in Progress Rufous-crowned Sparrow - Scott Morrison, The Nature Conservancy (San Diego). -

Introduction
INTRODUCTION UC-Stallcup TEXT.indd 1 1/23/14 11:30 AM This contribution to the California Natural History Guide Series of the University of California Press follows a long tradi- tion of books that explain, explore, and celebrate the natural riches of California and beyond. Our intent is to tell beginning birders, or curious naturalists, the how, what, when, where, and why of birding. Because birds are so mobile, some individuals of most spe- cies can wander far from their natal homes and appear any- where. Here we have tried to include only those species most likely to be seen along the coast, from Big Sur to the Oregon border. This is not a field guide to bird identification, but a field guide to the birds themselves. Birding is a word that encompasses many concepts. For some, the activity of searching for and observing birds is a clear window into the natural world, an affirmation of its beauty and its peacefulness. To others, birding is a delightful diversion from the hectic or perhaps boring daily routine of the mod- ern world— providing calm amid the chaos. Birds are nature’s ambassadors, connecting us through their ancient lineage to evolution’s astonishing creativity and offering us some guid- ance, through our study of their habitat needs, in our steward- ship of the Earth. Some people have found the wonders of birds to be the perfect antidote to sadness or loneliness, or a path to comradeship with kindred spirits; others consider the complex- ities of identification or behavior an intellectual challenge. -

12 GEO V 1921 No 57 Animals Protection and Game
12 GEO. V.] Animals Protection and Game. [1921, No. 57. 465 New Zealand. ANALYSIS. Title. PART IV. 1. Sbort Title and commencement. AC<JLDlATIZATION DIsTRICTS AND BOOIIIITIlIIS. S. Interpretation. 21. Acolimatization distriots. 22. ~ration of existing acclimatization looie· PART I. 23. Registration of societies formed after com· AlOMALII l'BOTBOTION. mencement of tbis Aot. 3. Certain animals to be absolutely protected. 24. Registered societies to be bodies oorporate. 4. PartiaJ protection of animals. 26. Alterations of rules to be approved by tbe 5. As to animals ceasing to be absolutely pro Minister. tected. 26. Annual balance-sheet, &0., to be forwarded to 6. Sanotuaries for imported and native game. Minister of Finance. 27. Wbere default made in forwarding balanoe 7. Land may be taken for sanotuaries, &c. sheet. 28. Vesting of animals in sooieti~. 29. Societies to notify Minister of imported PART IL animals tumed at large. Governor-General GAME. may vest in societies property in suoh animals. 8. Imported game and native ga.me. 9. Open seasons for imported and native game. PART V. O1fence to take or kill ga.me.during olose GENERAL. season. 30. Restriotion on importation, liberation, or 10. Notification as to oonditions on whioh open keeping of animals. Master, owner, &o.~ season deolared. of ship to prevent noxious reptiles or in- 11. No game to be trapped. Use of metal- sects from being landed in New Zealand. patched or metal-oased bullets unlawful. I Offenee. 12. Use of heavy guns unlawful. 31. Minister may authorize catching or taking of 13. Use of cylinders. silencers, and live decoys animals for certain purposes. -

Checklist: Birds of Andrew Molera State Park, Big Sur, California
Checklist: Birds of Andrew Molera State Park, Big Sur, California [ ] Red-throated Loon [ ] Cinnamon Teal [ ] * American Avocet [ ] ** Thick-billed Murre [ ] Pacific Loon [ ] * Northern Shoveler [ ] Greater Yellowlegs [ ] B Pigeon Guillemot [ ] Common Loon [ ] * Northern Pintail [ ] * Lesser Yellowlegs [ ] ** Marbled Murrelet [ ] Pied-billed Grebe [ ] Green-winged Teal [ ] * Solitary Sandpiper [ ] * Xantus' Murrelet [ ] Horned Grebe [ ] * Redhead [ ] Willet [ ] * Craveri's Murrelet [ ] Red-necked Grebe [ ] * Ring-necked Duck [ ] Wandering Tattler [ ] * Ancient Murrelet [ ] Eared Grebe [ ] * Greater Scaup [ ] Spotted Sandpiper [ ] Cassin's Auklet [ ] Western Grebe [ ] * Lesser Scaup [ ] Whimbrel [ ] Rhinoceros Auklet [ ] Clark's Grebe [ ] * Harlequin Duck [ ] * Long-billed Curlew [ ] * Tufted Puffin [ ] * Laysan Albatross [ ] Surf Scoter [ ] Marbled Godwit [ ] ** Horned Puffin [ ] Black-footed Albatross [ ] * White-winged Scoter [ ] * Ruddy Turnstone [ ] I Rock Dove [ ] Northern Fulmar [ ] * Black Scoter [ ] Black Turnstone [ ] B Band-tailed Pigeon [ ] Pink-footed Shearwater [ ] Common Goldeneye [ ] Surfbird [ ] ** White-winged Dove [ ] * Flesh-footed Shearwater [ ] * Barrow's Goldeneye [ ] * Red Knot [ ] B Mourning Dove [ ] Buller's Shearwater [ ] * Hooded Merganser [ ] Sanderling [ ] ** Common Ground-Dove [ ] Sooty Shearwater [ ] B Common Merganser [ ] Western Sandpiper [ ] ** Black-billed Cuckoo [ ] Black-vented Shearwater [ ] Red-breasted Merganser [ ] Least Sandpiper [ ] ** Yellow-billed Cuckoo [ ] Fork-tailed Storm-Petrel [ ] * Ruddy -

25 California Quail
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Grouse and Quails of North America, by Paul A. Johnsgard Papers in the Biological Sciences 5-8-1973 25 California Quail Paul A. Johnsgard University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/bioscigrouse Part of the Ornithology Commons Johnsgard, Paul A., "25 California Quail" (1973). Grouse and Quails of North America, by Paul A. Johnsgard. 27. https://digitalcommons.unl.edu/bioscigrouse/27 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Grouse and Quails of North America, by Paul A. Johnsgard by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. California Quail Cdllipepl. califomia (Shaw) 1798 (Lophortyx califomktls in A.0. U. Check-list) OTHER VERNACULAR NAMES ALIFORNIA partridge, Catalina quail, Codorniz ?T - Californiana, crested quail, San Lucas quail, San Quintin quail, topknot quail, valley quail. RANGE From southern Oregon and western Nevada south to the tip of Baja California. Introduced into southern British Columbia, Washington, Idaho, northern Oregon, and Utah. SUBSPECIES (ex A.O. U. Check-list) C. c. californica: Valley California quail. Resident from northern Oregon and western Nevada south to southern California and Los Coronados Islands of Baja California. Introduced in eastern Washington, central British Colum- bia, western Idaho, Oregon, Utah, and Colorado. C. c. catalinensis (Grinnell): Catalina Island California quail. Resident on Santa Catalina Island and introduced on Santa Rosa and Santa Cruz islands, southern California. -

Target Species Mapping for the Green Visions Plan
Target Species Habitat Mapping California Quail and Mountain Quail (Callipepla californica and Oreortyx pictus) Family: Phasianidae Order: Galliformes Class: Aves WHR #: B140 and B141 Distribution: California quail are found in southern Oregon, northern Nevada, California, and Baja California, and have been introduced in other states such as Hawaii, Washington, Idaho, Colorado, and Utah (Peterson 1961). In California, they are widespread but absent from the higher elevations of the Sierra Nevada, the Cascades, the White Mountains, and the Warner Mountains, and are replaced by the related Gambel’s quail (C. gambelii) in some desert regions (Peterson 1961, Small 1994). In southern California, they are found from the Coast Range south to the Mexican border, and occur as far east as the western fringes of the Mojave and Sonoran deserts, such as in the Antelope Valley (Garrett and Dunn 1981, Small 1994). California quail range from sea level to about 5000 ft (1524 meters; Stephenson and Calcarone 1999) Mountain quail are resident from northern Washington and northern Idaho, south through parts of Oregon, northwestern Nevada, California, and northern Baja California (Peterson 1961). In southern California, mountain quail are found in nearly all of the mountain ranges west of the deserts, including the southern Coast Ranges, from the Santa Lucia Mountains south through Santa Barbara and Ventura counties, and the Peninsular Ranges south to the Mexican border (Garrot and Dunn 1981, Small 1994). In the Transverse Ranges, a small population occurs in the western Santa Monica Mountains, and larger populations occur in the San Gabriel and San Bernardino Mountains (Small 1994). Mountain quail are found at elevations from below 2000 ft (610 meters) to over 9000 ft (2743 meters; Stephenson and Calcarone 1999). -

Quail-Friendly Plants of North-West Baja California
RANCHO SANTA ANA BOTANIC GARDEN OCCASIONAL PUBLICATIONS NUMBER 11 QUAIL-FRIENDLY PLANTS OF BAJA CALIFORNIA: AN EXPLORATION OF THE FLORA OF THE SANTO TOMÁS, SAN VICENTE, SAN JACINTO, AND SAN QUINTÍN VALLEYS, CORE HABITAT FOR THE CALIFORNIA QUAIL (CALLIPEPLA CALIFORNICA SUBSP. PLUMBEA) Sula Vanderplank Contributors John Trendler is Curator of Visual Jim Folsom is Director of Huntington Resources at Scripps College and Graphic Botanical Gardens. Jim is the primary and Information Design Consultant. His collaborator on this project. His layout and design work was assisted by assistance with the project development, Winona Bechtle and Nicole Frazer. and the contributions of his employees, made this guide possible. John Macdonald is Photographer at the Barbara Eisenstein is a Native Plant Seed Bank of Rancho Santa Ana Botanic Garden Consultant from Pasadena. In Gardens. Co-author of “Processing Seeds addition to her participation in field work of California Native Plants”, John has and via image contributions, Barbara contributed seed images for most plants was heavily involved in image selection, in this guide. color correction and formatting. The following people contributed images to this publication after their participation in field work: Cover photos: Quail: (Nueva York, Baja California) Alan Harper © 2011 (alanharper.com) Landscape: (Eréndira, Baja California) Sula Vanderplank Cody Coyotee John Trager is Sean Lahmeyer is This work was made possible by the generous financial assistance of Club La Misión of SanVicente, Baja California, Mexico; the Howard is Curator of Desert Plant Conservation Miller Family Charitable Trust; and the Walter Lantz Foundation. Cost-sharing was graciously provided by Rancho Santa Ana Botanic Conservation Collections at Specialist at the Garden and Huntington Botanical Gardens. -

Grant Report California Quail
Grant Report California Quail Translocation from Idaho to Texas California Quail: Translocation from Idaho to Texas Final Report September 2020 Prepared by: Kelly S. Reyna, Jeffrey G. Whitt, Sarah A. Currier, Shelby M. Perry, Garrett T. Rushing, Jordan T. Conley, Curt A. Vandenberg, and Erin L. Moser. The Quail Research Laboratory, College of Agricultural Sciences and Natural Resources, Texas A&M University Commerce 1 TABLE OF CONTENTS TABLE OF FIGURES AND TABLES ................................................................................ 3 BRIEF: ................................................................................................................... 5 INTRODUCTION ................................................................................................... 7 RESEARCH GOALS .................................................................................................... 8 PREDATOR IMPACTS ON TRANSLOCATED QUAIL ........................................................... 8 PREDATOR AVOIDANCE BEHAVIOR OF TRANSLOCATED QUAIL ...................................... 8 IMPACTS OF TEXAS HEAT ON VALLEY QUAIL DEVELOPMENT ........................................... 9 DEVELOPMENTAL TRAJECTORY OF CALIFORNIA VALLEY QUAIL .................................... 10 TRANSLOCATION WEIGHT LOSS ................................................................................ 10 PROJECT DESIGN............................................................................................... 11 MATERIAL AND METHODS ................................................................................ -
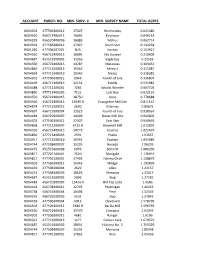
Account Parcel No. Min. Surv. # Min. Survey Name Total Acres
ACCOUNT PARCEL NO. MIN. SURV. # MIN. SURVEY NAME TOTAL ACRES N004302 477506300012 17307 Wednesday 0.041486 N004550 456722400015 16095 Keystone 0.046553 N004329 456520400006 16089 Walrus 0.062714 N004302 477506300012 17307 South Star 0.133258 R001186 477506207001 N/A Ironton 0.153927 N004550 456722400015 16095 Sky Scraper 0.219906 N004687 451519100002 11956 Eagle Eye 0.22526 N004550 456722400015 14787 Matanzas 0.303452 N004840 477713200013 19343 Selma 2 0.311987 N004924 477713300019 19343 Mezzo 0.328382 N004302 477506300012 6946 Fourth of July 0.336807 N004469 456717400015 12151 Estella 0.531982 N004488 477712100001 7265 Schultz Wonder 0.607702 N004830 477713400005 7121 Lost Boy 0.622125 N004550 456722400015 18750 Gore 0.778688 N004550 456722400015 12949 B Evangeline Mill Site 0.811167 N004874 477713300012 2690 Killarney 0.89675 N004697 456719100004 13523 Fourth of July 0.938567 N004484 456720300007 14499 Blazer Mill Site 0.944802 N004302 477506300012 17307 East Star 0.949663 N004868 477712200007 6712 B Brownell Mill 1.012899 N004550 456722400015 14773 Cosmos 1.021479 N004830 477713400005 2591 Puzzle 1.03637 N004917 477713300016 19335 Captain 1.055989 N004744 477508400007 15205 Nevada 1.06235 N004472 451519300008 6956 Bennett 1.086269 N004817 477701100001 7020 Marigold 1.126919 N004817 477701100001 17493 Yakima Chief 1.158879 N004302 477506300012 19343 Wedge 1.192899 N004459 477506300004 2629 Allen 1.22157 N004431 477508300005 18626 Primrose 1.33017 N004687 451519100002 5906 King 1.37281 N004484 456720300007 13424 A Hill Top Lode 1.4586 N004464 456728100012 12749 Ptarmigan 1.46463 N004738 456716300004 16494 Ymir 1.52425 N004335 456729200003 5532 Gap 1.57842 N004459 477506300004 6913 Cleveland 1.578799 N004302 477506300012 2386 B Barilla Mill 1.596539 N004550 456722400015 15370 Compass 1.61097 N004302 477506300012 4681 Ranger 1.6196 R006562 477713300013 1177 Carbon Lake 1.679579 N004687 451519100002 18031 Hultona No. -

Landbird Inventory for Devil's Postpile
Landbird Inventory for Devil’s Postpile National Monument Final Report Rodney B. Siegel and Robert L. Wilkerson with collaboration from Sacha K. Heath The Institute for Bird Populations P.O. Box 1346 Point Reyes Station, CA 94956-1346 April 7, 2004 Cooperative Agreement H9471011196 Table of Contents List of Tables................................................................................................................... iii List of Figures ................................................................................................................. iii Summary ..........................................................................................................................iv Acknowledgments.............................................................................................................v Introduction .......................................................................................................................1 Methods.............................................................................................................................2 Sampling strategy..................................................................................................2 Field methods........................................................................................................2 Crew training and testing ......................................................................................3 Data analysis .........................................................................................................3