Effects of Continuous Cropping on Bacterial Community Structure in Rhizospheric Soil of Sweet Potato
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ps TOILETRY CASE SETS ACROSS LIFE and DEATH in EARLY CHINA (5 C. BCE-3 C. CE) by Sheri A. Lullo BA, University of Chicago
TOILETRY CASE SETS ACROSS LIFE AND DEATH IN EARLY CHINA (5th c. BCE-3rd c. CE) by Sheri A. Lullo BA, University of Chicago, 1999 MA, University of Pittsburgh, 2003 Submitted to the Graduate Faculty of Arts & Sciences in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Pittsburgh 2009 Ps UNIVERSITY OF PITTSBURGH FACULTY OF ARTS & SCIENCES This dissertation was presented by Sheri A. Lullo It was defended on October 9, 2009 and approved by Anthony Barbieri-Low, Associate Professor, History Dept., UC Santa Barbara Karen M. Gerhart, Professor, History of Art and Architecture Bryan K. Hanks, Associate Professor, Anthropology Anne Weis, Associate Professor, History of Art and Architecture Dissertation Advisor: Katheryn M. Linduff, Professor, History of Art and Architecture ii Copyright © by Sheri A. Lullo 2009 iii TOILETRY CASE SETS ACROSS LIFE AND DEATH IN EARLY CHINA (5th c. BCE-3rd c. CE) Sheri A. Lullo, PhD University of Pittsburgh, 2009 This dissertation is an exploration of the cultural biography of toiletry case sets in early China. It traces the multiple significances that toiletry items accrued as they moved from contexts of everyday life to those of ritualized death, and focuses on the Late Warring States Period (5th c. BCE) through the Han Dynasty (206 BCE-220 CE), when they first appeared in burials. Toiletry case sets are painted or inlaid lacquered boxes that were filled with a variety of tools for beautification, including combs, mirrors, cosmetic substances, tweezers, hairpins and a selection of personal items. Often overlooked as ordinary, non-ritual items placed in burials to comfort the deceased, these sets have received little scholarly attention beyond what they reveal about innovations in lacquer technologies. -

Atmospheric Environment 141 (2016) 20E29
Atmospheric Environment 141 (2016) 20e29 Contents lists available at ScienceDirect Atmospheric Environment journal homepage: www.elsevier.com/locate/atmosenv Trans-boundary aerosol transport during a winter haze episode in China revealed by ground-based Lidar and CALIPSO satellite * Kai Qin a, Lixin Wu a, , Man Sing Wong b, Husi Letu d, Mingyu Hu a, Hongmei Lang a, Shijie Sheng c, Jiyao Teng a, Xin Xiao a, Limei Yuan a a School of Environment Science and Spatial Informatics, China University of Mining and Technology, Xuzhou, China b Department of Land Surveying and Geo-Informatics, The Hong Kong Polytechnic University, Kowloon, Hong Kong c Wuxi CAS Photonics Corporation, Wuxi, China d Institute of Remote Sensing and Digital Earth, Chinese Academy of Sciences, Beijing, China highlights A trans-boundary transport of aerosols during a large-area haze episode in China during 3e5 January 2015 was investigated. Pollutants moving from Hebei, Henan, and Hubei probably contributed to the haze pollution in Shandong and Jiangsu. A considerable amount of total optical depth below 3 km (46% in average) was contributed by the external aerosol layers Haze transports from North China Plain to East China could be a common phenomenon influenced by the winter monsoon. article info abstract Article history: By employing PM2.5 observation data, ground-based lidar measurements, MODIS and CALIPSO satellite Received 28 January 2016 images, meteorological data, and back trajectories analysis, we investigate a trans-boundary transport of Received in revised form aerosols during a large-area haze episode in China during 3e5 January 2015. The ground-based lidar 30 May 2016 observations indicated similar episodes of external aerosols passing through and mixing into three East Accepted 16 June 2016 China cities. -

Tombstone Carvings from AD 86
Tombstone Carvings from AD 86 Did Christianity Reach China In the First Century? † Wei-Fan Wang Retired Professor Nanjing Theological Seminary 1 This study, carried out as part of the Chaire de recherche sur l’Eurasie (UCLy), will be issued in English in the volume The Acts of Thomas Judas, in context to be published in the Syro- Malabar Heritage and Research Centre collection, Kochin (Indian Federation) 2 Table of contents I. The Gospel carved on stone ......................................................................................... 5 Fig. 1 situation of Xuzhou .............................................................................................. 5 Fig. 2 : The phoenixes and the fish ................................................................................ 6 II. The Creation and the Fall ........................................................................................... 7 Fig. 3: Domestic animals ................................................................................................ 7 Fig. 4: temptation of Eve ................................................................................................ 7 Fig. 5: The cherubim and the sword ............................................................................... 8 ..................................................................................................................................... 9 Fig. 6: The exit of the Eden garden ................................................................................ 9 Fig. 7: Pillar of ferocious -

Congressional-Executive Commission on China Annual
CONGRESSIONAL-EXECUTIVE COMMISSION ON CHINA ANNUAL REPORT 2007 ONE HUNDRED TENTH CONGRESS FIRST SESSION OCTOBER 10, 2007 Printed for the use of the Congressional-Executive Commission on China ( Available via the World Wide Web: http://www.cecc.gov VerDate 11-MAY-2000 01:22 Oct 11, 2007 Jkt 000000 PO 00000 Frm 00001 Fmt 6011 Sfmt 5011 38026.TXT CHINA1 PsN: CHINA1 2007 ANNUAL REPORT VerDate 11-MAY-2000 01:22 Oct 11, 2007 Jkt 000000 PO 00000 Frm 00002 Fmt 6019 Sfmt 6019 38026.TXT CHINA1 PsN: CHINA1 CONGRESSIONAL-EXECUTIVE COMMISSION ON CHINA ANNUAL REPORT 2007 ONE HUNDRED TENTH CONGRESS FIRST SESSION OCTOBER 10, 2007 Printed for the use of the Congressional-Executive Commission on China ( Available via the World Wide Web: http://www.cecc.gov U.S. GOVERNMENT PRINTING OFFICE 38–026 PDF WASHINGTON : 2007 For sale by the Superintendent of Documents, U.S. Government Printing Office Internet: bookstore.gpo.gov Phone: toll free (866) 512–1800; DC area (202) 512–1800 Fax: (202) 512–2104 Mail: Stop IDCC, Washington, DC 20402–0001 VerDate 11-MAY-2000 01:22 Oct 11, 2007 Jkt 000000 PO 00000 Frm 00003 Fmt 5011 Sfmt 5011 38026.TXT CHINA1 PsN: CHINA1 VerDate 11-MAY-2000 01:22 Oct 11, 2007 Jkt 000000 PO 00000 Frm 00004 Fmt 5011 Sfmt 5011 38026.TXT CHINA1 PsN: CHINA1 CONGRESSIONAL-EXECUTIVE COMMISSION ON CHINA LEGISLATIVE BRANCH COMMISSIONERS House Senate SANDER M. LEVIN, Michigan, Chairman BYRON DORGAN, North Dakota, Co-Chairman MARCY KAPTUR, Ohio MAX BAUCUS, Montana TOM UDALL, New Mexico CARL LEVIN, Michigan MICHAEL M. HONDA, California DIANNE FEINSTEIN, California TIM WALZ, Minnesota SHERROD BROWN, Ohio CHRISTOPHER H. -
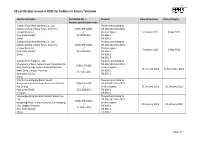
CE Certificates Issued in 2021 for Holders in China / Vietnam
CE certificates issued in 2021 for holders in China / Vietnam Certificate holder Certificate No. / Product Date of issuance Date of expiry Product specification code Xuzhou Yizun New Material Co., Ltd. Plywood according to Industrial Park, Hegou Town, Xinyi City, 0766-CPR-429/4 EN 636:2012+A1:2015 Jiangsu Province / Product types: 5 January 2021 9 May 2021 Post code 221439 2117181-009 EN 636-1 China EN 636-2 Xuzhou Yizun New Material Co., Ltd. Plywood according to Industrial Park, Hegou Town, Xinyi City, 0766-CPR-430/4 EN 636:2012+A1:2015 Jiangsu Province / Product types: 5 January 2021 9 May 2021 Post code 221439 2117181-010 EN 636-1 China EN 636-2 EN 636-3 Xuzhou Kinri Trade Co., Ltd. Plywood according to Shengyang Village, Sanpu Town, Tongshan Dis- EN 636:2012+A1:2015 0766-CPR-564 trict, Xuzhou High-tech Industrial Develop- Product types: / 11 January 2021 24 November 2021 ment Zone, Jiangsu Province EN 636-1 2121007-005 Post code 221112 EN 636-2 China Viet Nam Hai Duong Baifar Wood Plywood according to Nam Sach Industrial Zone, Nam Sach District, 0766-CPR-565 EN 636:2012+A1:2015 Hai Duong / Product types: 11 January 2021 10 January 2022 Post code 03000 2121008-001 EN 636-1 Vietnam EN 636-2 LianyungangChanta International Wood Cov Plywood according to Ltd. EN 636:2012+A1:2015 0766-CPR-485/2 Kangpeng Plaza, Lianyun District, Lianyungang Product types: / 26 January 2021 20 January 2022 City, Jiangsu Province EN 636-1 2119007-003 Post code 222000 EN 636-2 China EN 636-3 Page 1 of 7 CE certificates issued in 2021 for holders in China / Vietnam Certificate holder Certificate No. -

Study of the Allocation of Regional Flood Drainage Rights In
International Journal of Environmental Research and Public Health Article Study of the Allocation of Regional Flood Drainage Rights in Watershed Based on Entropy Weight TOPSIS Model: A Case Study of the Jiangsu Section of the Huaihe River, China Kaize Zhang 1,2, Juqin Shen 3, Han Han 1,* and Jinglai Zhang 4 1 Business School, Hohai University, Nanjing 211100, China; [email protected] 2 Department of Ecosystem Science and Management, The Pennsylvania State University, State College, PA 16802, USA 3 College of Agricultural Engineering, Hohai University, Nanjing 210098, China; [email protected] 4 Department of Chemistry, College of Chemistry and Chemical Engineering, Henan University, Kaifeng 475001, China; [email protected] * Correspondence: [email protected] Received: 13 June 2020; Accepted: 10 July 2020; Published: 13 July 2020 Abstract: During the flood season, various regions in a watershed often have flood drainage conflicts, when the regions compete for flood drainage rights (FDR). In order to solve this problem, it is very necessary to study the allocation of FDR among various regions in the watershed. Firstly, this paper takes fairness, efficiency and sustainable development as the allocation principles, and comprehensively considers the differences of natural factors, social development factors, economic development factors and ecological environment factors in various regions. Then, an indicator system for allocation of FDR among regions in the watershed is established. Secondly, an entropy weight Technique for Order Preference by Similarity to Ideal Solution (TOPSIS) model is used to construct the FDR allocation model among regions in the watershed. Based on a harmony evaluation model, a harmony evaluation and comparison are carried out on the FDR allocation schemes under three different allocation principles. -

A Comprehensive Evaluation of the Construction of Innovation Cities In
International Conference on Applied Science and Engineering Innovation (ASEI 2015) A Comprehensive Evaluation of the Construction of Innovation Cities in Xuzhou City Based on Principal Component Analysis Rong xiaohong Institute of information management, Xuzhou College of IndustrialTechnology, Xuzhou, 221140, China. [email protected] Keywords: innovative city; evaluation index; principal component analysis; Abstract. The evaluation of innovation cities assesses the realization level of innovative cities through the evaluation of the comprehensive innovative level. In this paper, the author builds an innovative city competitiveness evaluation index system based on the theoretical analysis, and evaluates Xuzhou city and other 12 prefecture-level cities the innovation capacity through principal component analysis. Alterations made recommendations based on the evaluation results, in order to provide the ability to provide innovative Xuzhou basis for decision making. 1. Introduction In the 21st century, innovation has become the first driver of a country or region’s economic development. Thus "innovative city" has become the focus of academic at home and abroad. Chard Florida[1] has done the city innovation ability evaluation of the United States from the empirical aspects, he adopts the index mainly includes high-tech, innovative, and human resources. EIS[2] thought that an core indicators of innovative city innovation ability included technology, talent and tolerance, which thus created the innovation capability index evaluation method.In the domestic, from the subjective and objective view, Fengxia[3] gives a new urban construction process of evaluation index should at least include ideas, the level of science and technology, system, macro environment and cultural atmosphere and so on five categories of innovation index. -

A Reconsideration of the Leilou – Longbian Debate: a Continuation of Research by Nishimura Masanari
asian review of world histories 5 (2017) 28–52 A Reconsideration of the Leilou – Longbian Debate: A Continuation of Research by Nishimura Masanari Phạm Lê Huy Vietnam National University-Hanoi [email protected] Abstract In 111 BCE, the Han Dynasty destroyed the Nanyue Kingdom and put the region under the Jiaozhi Commandery. The headquarters of the Jiaozhi Commandery was originally Miling, but was later moved to Leilou and then to Longbian. There have been many hypotheses regarding the location of Leilou and Longbian. In particular, many scholars have identified the remains of the old citadel (Lũng Khê Citadel) located in Thuận Thành District (Bắc Ninh, Vietnam) with the Leilou Citadel. Based on archaeological evidence, the late Dr. Nishimura Masanari advanced a new hypothesis that these remains were not the citadel of Leilou, but that of Longbian. This article will review historical documents about Leilou and Longbian and introduce two inscriptions recently discovered in Bắc Ninh Province to provide further support for Nishimura’s hypothesis. Keywords Jiaozhi Commandery – Leilou – Longbian – Lũng Khê citadel – Nishimura Masanari Literature Review In 111 BCE, the Han Dynasty (206 BCE–220 CE) destroyed the Kingdom of Nanyue and annexed its territory. Inheriting the administrative system of Nanyue, the Han put the region of Nanyue under the Jiaozhi Commandery (交趾郡). The headquarters of the Jiaozhi Commandery was originally Miling (麋泠, Vietnamese: Mê Linh), but was later moved to Leilou (羸軻, Vietnamese: Luy Lâu) and then to Longbian (龍編, Vietnamese: Long Biên). So far, there © koninklijke brill nv, leiden, 2�17 | doi 10.1163/22879811-12340004Downloaded from Brill.com09/25/2021 04:28:21AM via free access A Reconsideration of the Leilou – Longbian Debate 29 have been several hypotheses concerning the location of the Leilou and Longbian citadels. -

Region Provinces/Municipality City North Beijing Beijing Tianjin Tianjin Hebei Cangzhou Langfang Qinhuangdao Shijiazhuang Baodin
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) BMJ Open Appendix Table 1: Participants’ geographic distribution (26 provinces, 100 cities) Region Provinces/Municipality City Beijing Beijing Tianjin Tianjin Cangzhou Langfang North Qinhuangdao Hebei Shijiazhuang Baoding Tangshan Shanghai Shanghai Nanjing Zhenjiang Yangzhou Suqian Xuzhou Jiangsu Changzhou Wuxi Taizhou Suzhou Lianyungang Nantong Hangzhou Huzhou Wenzhou Lishui East Zhejiang Ningbo Jiaxing Jinhua Shaoxing Taizhou Maanshan Hefei Anhui Xuancheng Huainan Fuyang Xiamen Fuzhou Fujian Sanming Quanzhou Shangrao Jiangxi Ganzhou Nanchang Hall BJ, et al. BMJ Open 2021; 11:e048012. doi: 10.1136/bmjopen-2020-048012 BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) BMJ Open Jiujiang Jinan Linyi Qingdao Shandong Weifang Liaocheng Weihai Taian Luoyang Zhengzhou Henan Shangqiu Anyang Nanyang Huangshi Wuhan Central Jingzhou Hubei Xiangyang Yichang Xianning Changsha Hunan Hengyang Xiangtan Guangzhou Foshan Shenzuo Zhongshan Guangdong Zhaoqing Yangjiang Dongzuo South Huizhou Zhuhai Yulin Guangxi Nanning Hainan Haikou Changde Hongkong Hongkong Chengdu Yibin Zigong Sichuan Southwest Mianyang Dazhou Neijiang Guizhou Guiyang Hall BJ, et al. BMJ Open 2021; 11:e048012. doi: 10.1136/bmjopen-2020-048012 -

Artists of Traditional Chinese Paintings
Artists of Traditional Chinese Paintings: 马奉信 Ma Fengxin Ma Fengxin, born in Xuzhou, Jiangsu Province in May 1942, studied under Mr. Wang Guanzhong when he was young, and graduated from the Fine Arts of Nanjing Normal University. During his study, he was instructed by Lyu Sibai, Fu Baoshi and Yang Jianhou. Owing to his excellent grades when graduating, he furthered his study in the college for another two years. He is a member of Chinese Calligraphers Association, member of China Artists Association, member of China Canglang Book Society, librarian of Jiangsu Provincial Literature and History Museum, and a standing director of Xu Beihong Research Association of Jiangsu Province. He used to be a member of the Sixth, Seventh and Eighth CPPCC of Jiangsu Province, dean of Xuzhou Painting and Calligraphy Institute, vice chairman of Xuzhou Municipal Federation of Literary and Art Circles, and a distinguished professor of Fine Arts School of Jiangsu Normal University. 黄秉乙 Huang Bingyi Huang Bingyi, born in 1941, styled himself Ye Qing. In 1974, he took Mr. Kong Zhongqi as his teacher and specialized in landscape painting. In 1985, he entered the senior graduate class Chinese painting department of China Academy of Art and taught by Mr. Lu Yanshao. He is a member of China Hue Art Association, senior painter of Jiangsu Guofeng Painting and Calligraphy Institute, adjunct professor of Shandong Art Institute, and a painter of Xuzhou Qinxin Painting and Calligraphy Institute. 杨正伟 Yang Zhengwei Yang Zhengwei (Pseudonym:Zhi Hu) was born in 1948. He is currently a member of the China Artists Association, a national-level artist, an honorary dean of Painting and Calligraphy of Xuzhou Municipal Committee of China Association for Promoting Democracy, and the president of Xichu Landscape Painting Association. -

Can Higher Education, Economic Growth and Innovation Ability Improve Each Other?
sustainability Article Can Higher Education, Economic Growth and Innovation Ability Improve Each Other? Haiying Xu 1 , Wei-Ling Hsu 1,* , Teen-Hang Meen 2 and Ju Hua Zhu 3 1 School of Urban and Environmental Science, Huaiyin Normal University, Huai’an 223300, China; [email protected] 2 Department of Electronic Engineering, National Formosa University, Yunlin 632, Taiwan; [email protected] 3 Department of Civil, Environmental and Architectural Engineering, Korea University, Seoul 02841, Korea; [email protected] * Correspondence: [email protected] Received: 25 February 2020; Accepted: 22 March 2020; Published: 23 March 2020 Abstract: This study argues that the coupling between higher education, economic growth, and innovation ability is of great significance for regional sustainable development. Through the experience of Jiangsu Province in China, this study establishes a coupling coordination evaluation index system and applies the coupling coordination model to evaluate interactive relationships among the three. It finds that during 2007–2017, the level of coupling of 13 prefecture-level cities in Jiangsu was increasing over time, which fully verified the previous scholars’ view that the three can improve each other over a long period. However, this study finds that there are obvious differences within Jiangsu. Inadequate investment in higher education has become a crucial constraint on sustainable economic growth in northern and central Jiangsu, which are backward regions of Jiangsu. By contrast, in southern Jiangsu, which is the advanced region of Jiangsu, although the resources of higher education are abundant the growth of innovation ability cannot support sustained economic growth well. Thus, the quality of higher education should be improved to meet the needs of the innovation-based economy. -

Attachment 1
Barcode:3893626-02 A-570-114 INV - Investigation - Foreign Producer/Exporter Address Email ANHUA IMPORTS AND EXPORTS NO.7 BUILDING WUHU EXPORT & PROCESSING ZONE Anhui n/a ANHUI LONGRUI GLASS Zhangii, Guiing Town, Quzhou City, Anhui Province n/a ASIA TRADE CONNECTION 1814 HUA RUAN RD YUSHAN TO WN, KUNSHAN n/a Built in China Narnping Street, Penghu Road No. 6, Building 3, 4th Floor Room 3, NanAn City, Chongqing, China [email protected] ; [email protected] CANGZHOU ROTER FADEN GLASS PRODUCTS ROO M 501, UNIT 2, DORMITORY BLOCK 19, DONGSU, JIASHUYUAN, CangZhou City,Hebei Province,China h1ho_office_dz@ 126 .com CHANGYU GLASS East Jiangsu Road,Qixia Economic Development Zone,Shandong,China [email protected] CHOICEST INTERNATIONAL 8f, 447-1, PATE RD., SEC. 2, Shenzhen [email protected] East Asia Glass Limited 63739 street songiiang shanghai, China [email protected] FENGYANG HUA ZHONG GLASS Mentai Industrial Park, Chuzhou, Anhui, China (233100) n/a FUJIAN HUAXING GLASS Xiuyu Industrial Park, Hushi, Putian, Fujian, China [email protected] Guangdong huaxing glass co., LTD Luo village zhuang industrial zone,nanhai district, foshan city, guangdong province, China [email protected] GUANGZHOU IDEALPAK BUSINESS Room 102 NO.328, LiangTian North Road, Zhongluotan Town, Baiyun District [email protected] HAIMEN SANLONG GLASS PRODUCTS No.15 Desan Road Desheng Town Haimen City Jiangsu Province [email protected]; [email protected]; [email protected] HAPPYANN CRAFTS Buildingl09, Block 3, Square Gold Coast Rd.87, Cuiwen Road [email protected] Guoda Quancheng, No. 168 Donggang Road, Yuhua District, Shiiiazhuang City, Hebei Province, Shiiiazhuang, Hebei Anyu Glass Products Co., Ltd.