Phytoplankton As Index of Water Quality with Reference to Pollution of the Ganga River from Munger to Manihari, Bihar (India)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
AC with District Dist
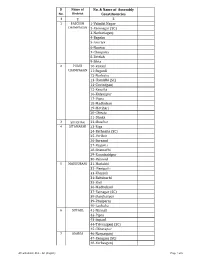
Sl Name of No. & Name of Assembly No. District Constituencies 1 2 3 1 PASCHIM 1-Valmiki Nagar CHAMPARAN 2-Ramnagar (SC) 3-Narkatiaganj 4-Bagaha 5-Lauriya 6-Nautan 7-Chanpatia 8-Bettiah 9-Sikta 2 PURVI 10-Raxaul CHAMPARAN 11-Sugauli 12-Narkatia 13-Harsidhi (SC) 14-Govindganj 15-Kesaria 16-Kalyanpur 17-Pipra 18-Madhuban 19-Motihari 20-Chiraia 21-Dhaka 3 SHEOHAR 22-Sheohar 4 SITAMARHI 23-Riga 24-Bathnaha (SC) 25-Parihar 26-Sursand 27-Bajpatti 28-Sitamarhi 29-Runnisaidpur 30-Belsand 5 MADHUBANI 31-Harlakhi 32- Benipatti 33-Khajauli 34-Babubarhi 35-Bisfi 36-Madhubani 37-Rajnagar (SC) 38-Jhanjharpur 39-Phulparas 40-Laukaha 6 SUPAUL 41-Nirmali 42-Pipra 43-Supaul 44-Triveniganj (SC) 45-Chhatapur 7 ARARIA 46-Narpatganj 47-Raniganj (SC) 48-Forbesganj AC with district Dist. - AC (English) Page 1 of 6 Sl Name of No. & Name of Assembly No. District Constituencies 1 2 3 49-Araria 50-Jokihat 51-Sikti 8 KISHANGANJ 52-Bahadurganj 53-Thakurganj 54-Kishanganj 55-Kochadhaman 9 PURNIA 56-Amour 57-Baisi 58-Kasba 59-Banmankhi (SC) 60-Rupauli 61-Dhamdaha 62-Purnia 10 KATIHAR 63-Katihar 64-Kadwa 65-Balrampur 66-Pranpur 67-Manihari (ST) 68-Barari 69-Korha (SC) 11 MADHEPURA 70-Alamnagar 71-Bihariganj 72-Singheshwar (SC) 73-Madhepura 12 SAHARSA 74-Sonbarsha (SC) 75-Saharsa 76-Simri Bakhtiarpur 77-Mahishi 13 DARBHANGA 78-Kusheshwar Asthan (SC) 79-Gaura Bauram 80-Benipur 81-Alinagar 82-Darbhanga Rural 83-Darbhanga 84-Hayaghat 85-Bahadurpur 86-Keoti 87-Jale 14 MUZAFFARPUR 88-Gaighat 89-Aurai 90-Minapur 91-Bochaha (SC) 92-Sakra (SC) 93-Kurhani 94-Muzaffarpur 95-Kanti 96-Baruraj AC with district Dist. -

Katihar District, Bihar State
भूजल सूचना पुस्तिका कटिहार स्जला, बिहार Ground Water Information Booklet Katihar District, Bihar State के न्द्रीय भमू िजल िो셍 ड Central Ground water Board Ministry of Water Resources जल संसाधन िंत्रालय (Govt. of India) (भारि सरकार) Mid-Eastern Region िध्य-पर्वू ी क्षेत्र Patna पिना मसिंिर 2013 September 2013 1 PREPARED BY - Sri Raj Kumar Singh, AHG UNDER SUPERVISION OF - Dr. K.K.Singh, Sc-’D’ & Sri A. K. Agrawal, Sc-’D’ UPDATED By - Sri S.N.Dwivedi, Sc-C & Dr. Fakhre Alam, STA (Hg) 2 Ground Water Information Booklet Katihar District, Bihar State CONTENTS S.No TITLES PAGE NO. 1.0 Introduction 6-8 1.1 Administrative details 1.2 Basin/sub-basin, Drainage 1.3 Irrigation Practices 1.4 Studies/Activities by CGWB 2.0 Climate and Rainfall 8 3.0 Geomorphology and Soils 8 4.0 Ground Water Scenario 8-13 4.1 Hydrogeology 4.2 Ground Water Resources 4.3 Ground Water Quality 4.4 Status of Ground Water Development 5.0 Ground Water Management Strategy 13-15 5.1 Ground Water Development 5.2 Water Conservation and Artificial Recharge 6.0 Ground Water related issue and problems 15 7.0 Mass Awareness and Training Activity 15 8.0 Area Notified by CGWB/SGWA 16 9.0 Recommendations 16 FIGURE 1.0 Index Map of Katihar district 2.0 Hydrogeological map of Katihar district 3.0 Pre monsoon (May 2011) water level map of Katihar district 4.0 Post monsoon (November 2011) water level map of Katihar district 5.0 Ground Water Potential Map of Katihar district 6.0 Categorization of blocks & Artificial Recharge Prospects TABLE 1.0 Long term (Decadal), Annual and Seasonal water level fluctuation of Katihar district for year 2011 2.0 Block-wise ground water resources of Katihar district (As on 31st March 2009) 3 KATIHAR DISTRICT AT A GLANCE Sl. -

DISTRICT : Katihar
District District District District District Sl. No. Name of Husband's/Father,s AddressDate of Catego Full Marks Percent Choice-1 Choice-2 Choice-3 Choice-4 Choice-5 Candidate Name Birth ry Marks Obtained age (With Rank) (With Rank) (With Rank) (With Rank) (With Rank) DISTRICT : Katihar 1 KUMARI PUNAM SRI BALESHWAR c/o- sri baleshwar 01-Jan-85 BC 700 631 90.14 Banka (2) Bhagalpur (2) Munger (2) Khagaria (1) Katihar (1) BHARTIA MANDAL mandal vill - babudih post -bhurna via- bausi, banka. bihar pin code - 813119 2SARITA KUMARISRI ARVIND RAM c/o- sri arvind ram das 05-Feb-86 BC 700 607 86.71 Banka (4) Bhagalpur (5) Munger (6) Khagaria (2) Katihar (2) DAS vill- babudih post- bhurna via- basi, banka, bihar- 813119 3 BINA KUMARISRI RANJAY vill- rahimpur chaudhary 05-Mar-75 GEN 900 730 81.11 Khagaria (5) Begusarai (2) Samastipur (3) Purnia (3) Katihar (3) CHAUDHARY tola post- rahimpur distt- khagaria 4 UPASNA KUMARISRI SURENDRA c/o- sri om prakash 01-Mar-77 BC 900 719 79.89 Khagaria (6) Begusarai (4) Saharsa (3) Madhepura (1) Katihar (4) KUMAR ranjan ( advocate ) police station road khagaria, post + p.s.- khagaria 5 RENU KUMARI RAJ KISHOR vill-kwai 05-Jan-70 BC 700 558 79.71 Nalanda (9) Gaya (7) Jahanabad (8) Patna (10) Katihar (5) PRASAD po-dhobdhia ps-khodaging dis-nalanda pin-801303 6 BANITA BHARTISRI PERYAG SINHA village- rasulpur, post- 05-Jul-88 BC 700 537 76.71 Lakhisarai (21) Munger (27) Banka (17) Gaya (13) Katihar (6) baha choki, p.s.- medni choki, district- lakhisarai 7 BIBHA BHARTISRI NIRAJ KUMAR w/o- sri niraj kumar 05-Jan-78 BC 900 690 76.67 Banka (18) Bhagalpur (27) Munger (29) Katihar (7) Katihar (7) vill- kamardih post- giridhara distt- banka pin code- 813211 8 BIBHA BHARTISRI NIRAJ KUMAR w/o- sri niraj kumar 05-Jan-78 BC 900 690 76.67 Banka (18) Bhagalpur (27) Munger (29) Katihar (7) Katihar (7) vill- kamardih post- giridhara distt- banka pin code- 813211 9 REMA KUMARIRAGHVENDAR vill+p.o- padva, p.s- 10-Jan-74 GEN 800 612 76.5 Madhepura (2) Saharsa (4) Supaul (1) Purnia (5) Katihar (9) SHARMA murligunj, dist- madhepura, pincode- 852122. -

Annexure-V State/Circle Wise List of Post Offices Modernised/Upgraded
State/Circle wise list of Post Offices modernised/upgraded for Automatic Teller Machine (ATM) Annexure-V Sl No. State/UT Circle Office Regional Office Divisional Office Name of Operational Post Office ATMs Pin 1 Andhra Pradesh ANDHRA PRADESH VIJAYAWADA PRAKASAM Addanki SO 523201 2 Andhra Pradesh ANDHRA PRADESH KURNOOL KURNOOL Adoni H.O 518301 3 Andhra Pradesh ANDHRA PRADESH VISAKHAPATNAM AMALAPURAM Amalapuram H.O 533201 4 Andhra Pradesh ANDHRA PRADESH KURNOOL ANANTAPUR Anantapur H.O 515001 5 Andhra Pradesh ANDHRA PRADESH Vijayawada Machilipatnam Avanigadda H.O 521121 6 Andhra Pradesh ANDHRA PRADESH VIJAYAWADA TENALI Bapatla H.O 522101 7 Andhra Pradesh ANDHRA PRADESH Vijayawada Bhimavaram Bhimavaram H.O 534201 8 Andhra Pradesh ANDHRA PRADESH VIJAYAWADA VIJAYAWADA Buckinghampet H.O 520002 9 Andhra Pradesh ANDHRA PRADESH KURNOOL TIRUPATI Chandragiri H.O 517101 10 Andhra Pradesh ANDHRA PRADESH Vijayawada Prakasam Chirala H.O 523155 11 Andhra Pradesh ANDHRA PRADESH KURNOOL CHITTOOR Chittoor H.O 517001 12 Andhra Pradesh ANDHRA PRADESH KURNOOL CUDDAPAH Cuddapah H.O 516001 13 Andhra Pradesh ANDHRA PRADESH VISAKHAPATNAM VISAKHAPATNAM Dabagardens S.O 530020 14 Andhra Pradesh ANDHRA PRADESH KURNOOL HINDUPUR Dharmavaram H.O 515671 15 Andhra Pradesh ANDHRA PRADESH VIJAYAWADA ELURU Eluru H.O 534001 16 Andhra Pradesh ANDHRA PRADESH Vijayawada Gudivada Gudivada H.O 521301 17 Andhra Pradesh ANDHRA PRADESH Vijayawada Gudur Gudur H.O 524101 18 Andhra Pradesh ANDHRA PRADESH KURNOOL ANANTAPUR Guntakal H.O 515801 19 Andhra Pradesh ANDHRA PRADESH VIJAYAWADA -

Nandan Gupta. `Prak-Bibar` Parbe Samaresh Basu. Nimai Bandyopadhyay
BOOK DESCRIPTION AUTHOR " Contemporary India ". Nandan Gupta. `Prak-Bibar` Parbe Samaresh Basu. Nimai Bandyopadhyay. 100 Great Lives. John Cannong. 100 Most important Indians Today. Sterling Special. 100 Most Important Indians Today. Sterling Special. 1787 The Grand Convention. Clinton Rossiter. 1952 Act of Provident Fund as Amended on 16th November 1995. Government of India. 1993 Vienna Declaration and Programme of Action. Indian Institute of Human Rights. 19e May ebong Assame Bangaliar Ostiter Sonkot. Bijit kumar Bhattacharjee. 19-er Basha Sohidera. Dilip kanti Laskar. 20 Tales From Shakespeare. Charles & Mary Lamb. 25 ways to Motivate People. Steve Chandler and Scott Richardson. 42-er Bharat Chara Andolane Srihatta-Cacharer abodan. Debashish Roy. 71 Judhe Pakisthan, Bharat O Bangaladesh. Deb Dullal Bangopadhyay. A Book of Education for Beginners. Bhatia and Bhatia. A River Sutra. Gita Mehta. A study of the philosophy of vivekananda. Tapash Shankar Dutta. A advaita concept of falsity-a critical study. Nirod Baron Chakravarty. A B C of Human Rights. Indian Institute of Human Rights. A Basic Grammar Of Moden Hindi. ----- A Book of English Essays. W E Williams. A Book of English Prose and Poetry. Macmillan India Ltd.. A book of English prose and poetry. Dutta & Bhattacharjee. A brief introduction to psychology. Clifford T Morgan. A bureaucrat`s diary. Prakash Krishen. A century of government and politics in North East India. V V Rao and Niru Hazarika. A Companion To Ethics. Peter Singer. A Companion to Indian Fiction in E nglish. Pier Paolo Piciucco. A Comparative Approach to American History. C Vann Woodward. A comparative study of Religion : A sufi and a Sanatani ( Ramakrishana). -
S No Atm Id Atm Location Atm Address Pincode Bank
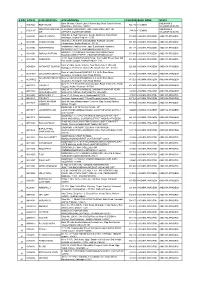
S NO ATM ID ATM LOCATION ATM ADDRESS PINCODE BANK ZONE STATE Bank Of India, Church Lane, Phoenix Bay, Near Carmel School, ANDAMAN & ACE9022 PORT BLAIR 744 101 CHENNAI 1 Ward No.6, Port Blair - 744101 NICOBAR ISLANDS DOLYGUNJ,PORTBL ATR ROAD, PHARGOAN, DOLYGUNJ POST,OPP TO ANDAMAN & CCE8137 744103 CHENNAI 2 AIR AIRPORT, SOUTH ANDAMAN NICOBAR ISLANDS Shop No :2, Near Sai Xerox, Beside Medinova, Rajiv Road, AAX8001 ANANTHAPURA 515 001 ANDHRA PRADESH ANDHRA PRADESH 3 Anathapur, Andhra Pradesh - 5155 Shop No 2, Ammanna Setty Building, Kothavur Junction, ACV8001 CHODAVARAM 531 036 ANDHRA PRADESH ANDHRA PRADESH 4 Chodavaram, Andhra Pradesh - 53136 kiranashop 5 road junction ,opp. Sudarshana mandiram, ACV8002 NARSIPATNAM 531 116 ANDHRA PRADESH ANDHRA PRADESH 5 Narsipatnam 531116 visakhapatnam (dist)-531116 DO.NO 11-183,GOPALA PATNAM, MAIN ROAD NEAR ACV8003 GOPALA PATNAM 530 047 ANDHRA PRADESH ANDHRA PRADESH 6 NOOKALAMMA TEMPLE, VISAKHAPATNAM-530047 4-493, Near Bharat Petroliam Pump, Koti Reddy Street, Near Old ACY8001 CUDDAPPA 516 001 ANDHRA PRADESH ANDHRA PRADESH 7 Bus stand Cudappa, Andhra Pradesh- 5161 Bank of India, Guntur Branch, Door No.5-25-521, Main Rd, AGN9001 KOTHAPET GUNTUR 522 001 ANDHRA PRADESH ANDHRA PRADESH Kothapeta, P.B.No.66, Guntur (P), Dist.Guntur, AP - 522001. 8 Bank of India Branch,DOOR NO. 9-8-64,Sri Ram Nivas, AGW8001 GAJUWAKA BRANCH 530 026 ANDHRA PRADESH ANDHRA PRADESH 9 Gajuwaka, Anakapalle Main Road-530026 GAJUWAKA BRANCH Bank of India Branch,DOOR NO. 9-8-64,Sri Ram Nivas, AGW9002 530 026 ANDHRA PRADESH ANDHRA PRADESH -
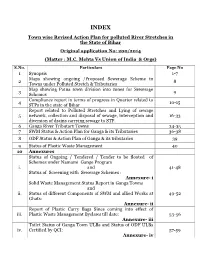
Town Wise Revised Action Plan for Polluted River Stretches in the State of Bihar Original Application No: 200/2014 (Matter : M.C
INDEX Town wise Revised Action Plan for polluted River Stretches in the State of Bihar Original application No: 200/2014 (Matter : M.C. Mehta Vs Union of India & Orgs) S.No. Particulars Page No 1 Synopsis 1-7 Maps showing ongoing /Proposed Sewerage Scheme in 2 8 Towns under Polluted Stretch & Tributaries Map showing Patna town division into zones for Sewerage 3 9 Schemes Compliance report in terms of progress in Quarter related to 4 10-15 STPs in the state of Bihar Report related to Polluted Stretches and Lying of sewage 5 network, collection and disposal of sewage, interception and 16-33 diversion of drains carrying sewage to STP. 6 Ganga River Tributary Towns 34-35 7 SWM Status & Action Plan for Ganga & its Tributaries 36-38 8 ODF Status & Action Plan of Ganga & its tributaries 39 9 Status of Plastic Waste Management 40 10 Annexures Status of Ongoing / Tendered / Tender to be floated of Schemes under Namami Gange Program i. and 41-48 Status of Screening with Sewerage Schemes : Annexure- i Solid Waste Management Status Report in Ganga Towns and ii. Status of different Components of SWM and allied Works at 49-52 Ghats: Annexure- ii Report of Plastic Carry Bags Since coming into effect of iii. Plastic Waste Management Byelaws till date: 53-56 Annexure- iii Toilet Status of Ganga Town ULBs and Status of ODF ULBs iv. Certified by QCI: 57-59 Annexure- iv 60-68 and 69 11 Status on Utilization of treated sewage (Column- 1) 12 Flood Plain regulation 69 (Column-2) 13 E Flow in river Ganga & tributaries 70 (Column-4) 14 Assessment of E Flow 70 (Column-5) 70 (Column- 3) 15 Adopting good irrigation practices to Conserve water and 71-76 16 Details of Inundated area along Ganga river with Maps 77-90 17 Rain water harvesting system in river Ganga & tributaries 91-96 18 Letter related to regulation of Ground water 97 Compliance report to the prohibit dumping of bio-medical 19 98-99 waste Securing compliance to ensuring that water quality at every 20 100 (Column- 5) point meets the standards. -

Ministry of Road Transport & Highways Government of India
Ministry of Road Transport & Highways Government of India Consultancy Services for preparation of Feasibility Study and Detailed Project Report for Improvement to Four laning /Two Lane with Paved shoulder of Four laning /Two Lane with Paved shoulder of (i) BH/WB Border – Amdabad – Manihari section of NH-131A including construction of Bridge over river Mahananda & Katihar Bypass and (ii) Raniganj Bypass between km 120 to 121 of NH-327E in Bihar on EPC / PPP Mode REQUEST FOR PROPOSAL (RFP) (International Competitive Bidding) March, 2016 1 INDEX Sl. No. Contents Page No. 1 Notice for inviting the proposals for Consultancy services 1 2 Letter of Invitation 2-15 3. Annex - I : List of Project along with Package Nos. 16 4. Data Sheet 17-22 5. Appendix - I : Terms of Reference 23-66 6. Supplement -I: Additional Requirements for Hill Roads 67-69 7. Supplement - II: Additional Requirements for Bridges 70-74 8 Supplement – III: Additional requirement for safety audit 75-77 9. Enclosure - I : Manning Schedule 78 10 Enclosure - II : Qualification Requirements of Key Personnel 79-82 11. Enclosure - III : Schedule for Submission of Reports and Documents 83 12. Appendix - II : Formats for Proof of Eligibility 84-90 13. Appendix - III : Formats for Technical Proposals 91-103 14. Appendix - IV : Formats for Financial Proposals 104-110 15. Appendix - V : Draft Contract Agreement 111-148 2 Ministry of Road Transport & Highways Government of India MORT&H intends to take up the Consultancy Services for preparation of Feasibility Study and Detailed Project Report for Improvement to Four laning/Two Lane with Paved shoulder (i) BH/WB Border – Amdabad – Manihari section of NH-131A including construction of Bridge over river Mahananda & Katihar Bypass and (ii) Raniganj Bypass between km 120 to 121 of NH-327E in Bihar on EPC / PPP Mode. -
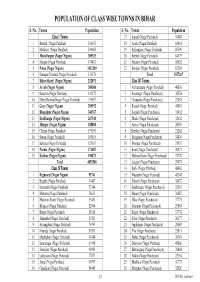
Population of Class Wise Towns in Bihar
POPULATION OF CLASS WISE TOWNS IN BIHAR S. No. Towns Population S. No. Towns Population Class I Towns 17 Supaul (Nagar Parishad) 54085 1 Bettiah (Nagar Parishad) 116670 18 Araria (Nagar Parishad) 60861 2 Motihari (Nagar Parishad) 100683 19 Kishanganj (Nagar Parishad) 85590 3 Muzaffarpur (Nagar Nigam) 305525 20 Beehat (Nagar Parishad) 64579 4 Hajipur (Nagar Parishad) 119412 21 Barauni (Nagar Parishad) 58628 5 Patna (Nagar Nigam) 1432209 22 Benipur (Nagar Parishad) 62203 6 Danapur Nizamat (Nagar Parishad) 131176 Total 1557267 7 Bihar Sharif (Nagar Nigam) 232071 Class III Towns 8 Arrah (Nagar Nigam) 203380 1 Narkatiaganj (Nagar Parishad) 40830 9 Sasaram (Nagar Parishad) 131172 2 Ramnagar (Nagar Panchayat) 38554 10 Dehri DalmiyaNagar (Nagar Parishad) 119057 3 Chanpatia (Nagar Panchayat) 22038 11 Gaya (Nagar Nigam) 389192 4 Raxaul (Nagar Parishad) 41610 12 Bhagalpur (Nagar Nigam) 340767 5 Sugauli (Nagar Panchayat) 31432 13 Darbhanga (Nagar Nigam) 267348 6 Dhaka (Nagar Panchayat) 32632 14 Munger (Nagar Nigam) 188050 7 Areraj (Nagar Panchayat) 20356 15 Chapra (Nagar Parishad) 179190 8 Sheohar (Nagar Panchayat) 21262 16 Siwan (Nagar Parishad) 109919 9 Bairgania (Nagar Panchayat) 34836 17 Saharsa (Nagar Parishad) 125167 10 Motipur (Nagar Panchayat) 21957 18 Purnia (Nagar Nigam) 171687 11 Kanti (Nagar Panchayat) 20871 19 Katihar (Nagar Nigam) 190873 12 Mahnar Bazar (Nagar Panchayat) 37370 Total 4853548 13 Lalganj (Nagar Panchayat) 29873 Class II Towns 14 Barh (Nagar Parishad) 48442 1 Begusarai (Nagar Nigam) 93741 15 Masaurhi (Nagar Parishad) 45248 -

CIN/BCIN Company/Bank Date of AGM FY-1 FY-2 FY-3 FY
Note: This sheet is applicable for uploading the particulars related to the unclaimed and unpaid amount pending with company. Make sure that the details are in accordance with the information already provided in e-form IEPF-2 L24223WB1947PLC015202 12-JUN-2020 CIN/BCIN Prefill Company/Bank DIC INDIA LIMITED Date of AGM FY-1 FY-2 FY-3 FY-4 FY-5 FY-6 FY-7 Sum of unpaid and unclaimed dividend 235240.00 283320.00 0.00 310984.00 328836.00 0.00 0.00 Number of underlying Shares 0.00 0.00 0.00 0.00 0.00 0.00 0.00 Sum of matured deposits 0.00 0.00 0.00 0.00 0.00 0.00 0.00 Sum of matured debentures 0.00 0.00 0.00 0.00 0.00 0.00 0.00 Sum of application money due for refund 0.00 0.00 0.00 0.00 0.00 0.00 0.00 Sum of interest on matured deposits 0.00 0.00 0.00 0.00 0.00 0.00 0.00 Sum of interest on matured debentures 0.00 0.00 0.00 0.00 0.00 0.00 0.00 Sum of interest on application money due for refund 0.00 0.00 0.00 0.00 0.00 0.00 0.00 Redemption amount of preference shares 0.00 0.00 0.00 0.00 0.00 0.00 0.00 Sales proceed for fractional shares 0.00 0.00 0.00 0.00 0.00 0.00 0.00 Sum of Other Investment Types 0.00 0.00 0.00 0.00 0.00 0.00 0.00 Validate Clear Is the shares Is the transfer from Proposed Date of Investment Investor Middle Investor Last Father/Husband Father/Husband Father/Husband Last DP Id-Client Id- Amount Joint Holder unpaid Investor First Name Address Country State District Pin Code Folio Number Investment Type transfer to IEPF PAN Date of Birth Aadhar Number Nominee Name Remarks (amount / Financial Year Name Name First Name Middle Name Name Account Number transferred Name suspense (DD-MON-YYYY) shares )under account any litigation. -

Si No. District Block Panchayat Village Popln
LIST OF URC ALLOTTED TO BANKS FOR OPENING OF BANKING OUTLETS BY 31.12.2017 SI NO. DISTRICT BLOCK PANCHAYAT VILLAGE POPLN. ALLOTTED TO 1 ARARIA FORBESGANJ AURAHI EAST Hardia 9260 ALLAHABAD BANK 2 ARWAL KAPRI KOCHAHASA PANCHAYAT Kochahsa 6409 ALLAHABAD BANK 3 BEGUSARAI BACHHWARA RANI-I Gopalpur 5094 ALLAHABAD BANK 4 BEGUSARAI BIRPUR NOULA Gopalpur 5094 ALLAHABAD BANK 5 BEGUSARAI BEGUSARAI CHILMIL Hardia 7185 ALLAHABAD BANK 6 BHOJPUR PIRO TAR Tar 6123 ALLAHABAD BANK 7 KATIHAR BARARI RAUNIA Rounia 9273 ALLAHABAD BANK 8 MADHUBANI LAUKAHA (KHUTAUNA) MAJHAURA Pathrahi 8365 ALLAHABAD BANK 9 MADHUBANI MADHUBANI KHAJURI Khajuri 5152 ALLAHABAD BANK 10 PATNA PALIGANJ MADHAMA MAKHMILPUR Makhmilpur 6392 ALLAHABAD BANK 11 SAHARSA SONBARSA ATALKHA Jamhra 9421 ALLAHABAD BANK 12 SHEIKHPURA Sheikhpura Kosra Kosra 6707 ALLAHABAD BANK 13 ARARIA BHARGAMA VISHAHARIYA Bisaria 9628 ALLAHABAD BANK 14 ARARIA FORBESGANJ MUSAHARI Musahari 5399 ALLAHABAD BANK 15 ARARIA NARPATGANJ KHABDAH Kandhaili 5012 ALLAHABAD BANK 16 ARARIA RANIGANJ DHAMA Dhama 6142 ALLAHABAD BANK 17 ARARIA SIKTY KAUAKOH Pothia 7723 ALLAHABAD BANK 18 NALANDA HILSA AKABARPUR Akbarpur 5305 ALLAHABAD BANK 19 NALANDA PARBALPUR SHIVNAGAR Bazidpur Khirauti 6533 ALLAHABAD BANK 20 NALANDA SILAO GORAWAN Gorawan 6282 ALLAHABAD BANK 21 VAISHALI BHAGWANPUR KARHARI Karhari 5424 ALLAHABAD BANK 22 VAISHALI CHEHRAKALA RASULPUR FATAH Rasulpur Fateh 5231 ALLAHABAD BANK 23 VAISHALI MAHUA FATAHPUR PAKRI Fatehpur Pakri 5778 ALLAHABAD BANK 24 VAISHALI RAJAPAKAR LAGURAO VILNUPUR Nagrawan 5160 ALLAHABAD BANK 25 MADHEPURA -

BLOCK DANDKHORA SL. No. PANCHAYAT NAME 1 SAUDIYA 2 DWASAY 3 BHAMRAILI 4 DANDKHORA 5 RAYPUR 6 MAHESHPUR BLOCK HASANGANJ SL
SUBDIVISION KATIHAR BLOCK KATIHAR SL. No. PANCHAYAT NAME 1 DALAN-WEST 2 DALAN-EAST 3 BHAWARA 4 KATIHAR 5 GARBHELI 6 DEHARIYA 7 SIRNIA-WEST 8 SIRNIA-EAST BLOCK DANDKHORA SL. No. PANCHAYAT NAME 1 SAUDIYA 2 DWASAY 3 BHAMRAILI 4 DANDKHORA 5 RAYPUR 6 MAHESHPUR BLOCK HASANGANJ SL. No. PANCHAYAT NAME 1 KALSAR 2 DHERUWA 3 JAGARNATHPUR 4 RAMPUR 5 BALUA BLOCK KORHA SL. No. PANCHAYAT NAME 1 RAJWARA 2 DIGHRI 3 KHERIA 4 PAWAI 5 KORHA 6 BAHARKHAL 7 CHANDWA 8 BINODPUR 9 MAKDAMPUR 10 RAMPUR 11 BISHANPUR 12 BAWANGANJ 13 SIMARIYA NORTH 14 SIMARIYA SOUTH 15 SANDALPUR 16 MADHURA 17 MAHINATHPUR 18 MUSAPUR 19 BISARIYA 20 FULBARIYA 21 BHATWARA 22 BASGADHA 23 RAUTARA BLOCK SAMELI SL. No. PANCHAYAT NAME 1 DUMAR 2 MALHARIYA 3 CHANDPUR EAST 4 CHANDPUR WEST 5 KHAIRA 6 CHAKLAMOLANAGAR 7 CHHOHAR 8 MURADPUR BLOCK FALKA SL. No. PANCHAYAT NAME 1 PIRMOKAM 2 RAHTA 3 MAGHELI 4 MORSANDA 5 HATHWARA 6 BHARSIYA 7 SALEHPUR 8 SOHTHA NORTH 9 SOHTHA SOUTH 10 GOVINDPUR 11 SHABDA 12 POTHIYA 13 BHANGHA BLOCK KURSELA SL. No. PANCHAYAT NAME 1 N.MURADPUR 2 S.MURADPUR 3 W.MURADPUR 4 E.MURADPUR 5 SHAHPUR DHARMI 6 JARLAHI BLOCK BARARI SL. No. PANCHAYAT NAME 1 WEST BARINAGAR 2 LAXMIPUR 3 BARARI 4 EAST BARINAGAR 5 GURUMELA 6 NORTHBHANDARTAL 7 SOUTHBHANDARTAL 8 KANT NAGAR 9 SUJAPUR 10 SISIYA 11 JAGDISHPUR 12 BISHANPUR 13 BAKIYA SUKHAY 14 MOHNA CHANDPUR 15 BAISA GOVINDPUR 16 ROUNIYA 17 SIKKAT 18 BARETA 19 SAKRAILI 20 DURGAPUR 21 SUKHASAN 22 KABAR BLOCK MANSAHI SL. No. PANCHAYAT NAME 1 MOHANPUR 2 CHITORIYA 3 SAHEBNAGAR 4 BHERMARA 5 MARANGI 6 KURETHA 7 FULHARA BLOCK PRANPUR SL.