Effects of Human Use of NCCP Reserves on Reptile and Mammal Species in San Diego
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Xenosaurus Tzacualtipantecus. the Zacualtipán Knob-Scaled Lizard Is Endemic to the Sierra Madre Oriental of Eastern Mexico
Xenosaurus tzacualtipantecus. The Zacualtipán knob-scaled lizard is endemic to the Sierra Madre Oriental of eastern Mexico. This medium-large lizard (female holotype measures 188 mm in total length) is known only from the vicinity of the type locality in eastern Hidalgo, at an elevation of 1,900 m in pine-oak forest, and a nearby locality at 2,000 m in northern Veracruz (Woolrich- Piña and Smith 2012). Xenosaurus tzacualtipantecus is thought to belong to the northern clade of the genus, which also contains X. newmanorum and X. platyceps (Bhullar 2011). As with its congeners, X. tzacualtipantecus is an inhabitant of crevices in limestone rocks. This species consumes beetles and lepidopteran larvae and gives birth to living young. The habitat of this lizard in the vicinity of the type locality is being deforested, and people in nearby towns have created an open garbage dump in this area. We determined its EVS as 17, in the middle of the high vulnerability category (see text for explanation), and its status by the IUCN and SEMAR- NAT presently are undetermined. This newly described endemic species is one of nine known species in the monogeneric family Xenosauridae, which is endemic to northern Mesoamerica (Mexico from Tamaulipas to Chiapas and into the montane portions of Alta Verapaz, Guatemala). All but one of these nine species is endemic to Mexico. Photo by Christian Berriozabal-Islas. amphibian-reptile-conservation.org 01 June 2013 | Volume 7 | Number 1 | e61 Copyright: © 2013 Wilson et al. This is an open-access article distributed under the terms of the Creative Com- mons Attribution–NonCommercial–NoDerivs 3.0 Unported License, which permits unrestricted use for non-com- Amphibian & Reptile Conservation 7(1): 1–47. -

Rationales for Animal Species Considered for Species of Conservation Concern, Sequoia National Forest
Rationales for Animal Species Considered for Species of Conservation Concern Sequoia National Forest Prepared by: Wildlife Biologists and Biologist Planner Regional Office, Sequoia National Forest and Washington Office Enterprise Program For: Sequoia National Forest June 2019 In accordance with Federal civil rights law and U.S. Department of Agriculture (USDA) civil rights regulations and policies, the USDA, its Agencies, offices, and employees, and institutions participating in or administering USDA programs are prohibited from discriminating based on race, color, national origin, religion, sex, gender identity (including gender expression), sexual orientation, disability, age, marital status, family/parental status, income derived from a public assistance program, political beliefs, or reprisal or retaliation for prior civil rights activity, in any program or activity conducted or funded by USDA (not all bases apply to all programs). Remedies and complaint filing deadlines vary by program or incident. Persons with disabilities who require alternative means of communication for program information (e.g., Braille, large print, audiotape, American Sign Language, etc.) should contact the responsible Agency or USDA’s TARGET Center at (202) 720-2600 (voice and TTY) or contact USDA through the Federal Relay Service at (800) 877-8339. Additionally, program information may be made available in languages other than English. To file a program discrimination complaint, complete the USDA Program Discrimination Complaint Form, AD-3027, found online at http://www.ascr.usda.gov/complaint_filing_cust.html and at any USDA office or write a letter addressed to USDA and provide in the letter all of the information requested in the form. To request a copy of the complaint form, call (866) 632-9992. -
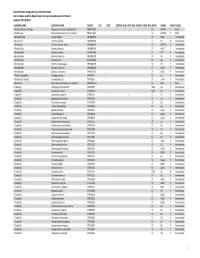
Element Status Designations by Common Name Arizona Game And
Element Status Designations by Common Name Arizona Game and Fish Department, Heritage Data Management System Updated: 10/15/2019 COMMON NAME SCIENTIFIC NAME ELCODE ESA DATE CRITHAB BLM USFS NESL MEXFED SGCN NPL SRANK GRANK TRACK TAXON A Arizona‐Mexican Orange Choisya arizonica var. amplophylla PDRUT02031 S2 G4TNR Y Plant A Balsamroot Balsamorhiza hookeri var. hispidula PDAST11041 S1 G5T3T5 Y Plant A Blueberry Bee Osmia ribifloris IIHYMA2570 S? G4G5 Y Invertebrate A Buckmoth Hemileuca grotei IILEW0M070 S? G4 N Invertebrate A Buckmoth Hemileuca grotei diana IILEW0M072 S? G4T3T4 Y Invertebrate A Bumble Bee Bombus centralis IIHYM24100 S? G4G5 Y Invertebrate A Bumble Bee Bombus fervidus IIHYM24110 S? G4? Y Invertebrate A Bumble Bee Bombus flavifrons IIHYM24120 S? G5 Y Invertebrate A Bumble Bee Bombus huntii IIHYM24140 S? G5 Y Invertebrate A Bumble Bee Bombus melanopygus IIHYM24150 S? G5 Y Invertebrate A Bumble Bee Bombus morrisoni IIHYM24460 S? G4G5 Y Invertebrate A Bumble Bee Bombus nevadensis IIHYM24170 S? G4G5 Y Invertebrate A Bushtail Caddisfly Gumaga griseola IITRI53010 S? G5 Y Invertebrate A Bushtailed Caddisfly Gumaga nigricula IITRI53020 S? G3G4 Y Invertebrate A Buttercup Ranunculus inamoenus var. subaffinis PDRAN0L1C3 S1 G5T1 YPlant A caddisfly Hydropsyche occidentalis IITRI25460 S2S3 G5 Y Invertebrate A caddisfly Hydropsyche oslari IITRIG6010 S2S3 G5 Y Invertebrate A caddisfly Lepidostoma apache IITRI64A10 S S1 G1 Y Invertebrate A Caddisfly Agapetus boulderensis IITRI33190 S? G5 Y Invertebrate A Caddisfly Alisotrichia arizonica IITRID7010 -
![Reptiles Squamata/Charinidae [ ] Lichanura Trivirgata Rosy Boa](https://docslib.b-cdn.net/cover/1134/reptiles-squamata-charinidae-lichanura-trivirgata-rosy-boa-2141134.webp)
Reptiles Squamata/Charinidae [ ] Lichanura Trivirgata Rosy Boa
National Park Service U.S. Department of the Interior Species Checklist for Mojave National Preserve (MOJA) This species list is a work in progress. It represents information currently in the NPSpecies data system and records are continually being added or updated by National Park Service staff. To report an error or make a suggestion, go to https://irma.nps.gov/npspecies/suggest. Scientific Name Common Name Reptiles Squamata/Charinidae [ ] Lichanura trivirgata rosy boa Squamata/Colubridae [ ] Arizona elegans glossy snake [ ] Chionactis occipitalis western shovel-nosed snake [ ] Coluber flagellum coachwhip [ ] Coluber taeniatus striped whipsnake [ ] Diadophis punctatus ring-necked snake [ ] Hypsiglena chlorophaea desert nightsnake [ ] Lampropeltis californiae California kingsnake [ ] Phyllorhynchus decurtatus spotted leaf-nosed snake [ ] Pituophis catenifer gopher snake [ ] Rhinocheilus lecontei long-nosed snake [ ] Salvadora hexalepis western patch-nosed snake [ ] Sonora semiannulata western groundsnake [ ] Tantilla hobartsmithi Smith's black-headed snake [ ] Trimorphodon biscutatus California lyresnake Squamata/Crotaphytidae [ ] Crotaphytus bicinctores Great Basin collared lizard [ ] Gambelia wislizenii long-nosed leopard lizard Squamata/Eublepharidae [ ] Coleonyx variegatus western banded gecko Squamata/Helodermatidae [ ] Heloderma suspectum gila monster Squamata/Iguanidae [ ] Dipsosaurus dorsalis desert iguana [ ] Sauromalus ater common chuckwalla [ ] Sceloporus occidentalis western fence lizard [ ] Sceloporus uniformis yellow-backed -

<Insert Month, Day and Year>
October 15, 2018 11414 Brittny Hummel Project Manager 2404 Wilshire Boulevard, Suite 9E Los Angeles, CA 90057 Subject: Biological Resources Letter Report for the 434, 438, 442, and 458 West James Street Project, City of Los Angeles, California Dear Ms. Hummel: This biological resources letter report provides the results of a biological resources assessment for the approximate 15,142.6 square-foot (0.35-acre) 434, 438, 442, and 458 West James Street Project property hereafter referred to as the “Project”, including a 500-foot buffer from the Project, hereafter referred to as the “study area”. The Project is located in the City of Los Angeles, in Los Angeles County, California (Assessor’s Parcel Numbers: 5452-011-013, 5452-011-004, 5452- 011-005, and 5452-011-006). Dudek understands that the Project proposes to construct four new single-family dwellings, each of which includes two-floors of living area over a garage. The northernmost single-family dwelling (APN: 5452-011-013) is located apart from the other three. As such, the Project site is comprised of two separate tracts of land separated by an intervening private residence. This letter report is intended to: (1) describe the existing conditions of biological resources within the Project site in terms of vegetation, flora, wildlife, and wildlife habitats; (2) quantify impacts to biological resources that would result from implementation of the proposed Project and describe those impacts in terms of biological significance in view of federal, state, and local laws and policies; and (3) recommend mitigation measures for impacts to sensitive biological resources, as applicable. -

October 2013
THE MONITOR NEWSLETTER OF THE HOOSIER HERPETOLOGICAL SOCIETY A non-profit organization dedicated to the education of its membership and the conservation of all amphibians and reptiles Volume 24 Number 10 October 2013 Welcome Hoosier Herpetological Society members! RENEWALS Pat Hammond October HHS meeting October 23rd 7:00 p.m. Holliday Park, Auditorium Speaker: Jim Horton Topic: "Snake Road Herping Adventures" Snake Road is located in the Southern tip of Illinois and is one of the most famous areas for "herpers" to explore. It is located between a large swamp and a bluff where the snakes hibernate during the winter.To visit there is like going back in time. Everyone that explores this area is impressed by its unspoiled pristine rugged beauty. Jim Horton, besides being our society's president and newsletter editor, is an experienced field "herper" and excellent wildlife photographer. He, along with other HHS members, have taken several trips to snake road. I have only been able to go to Snake Road once but it is a trip I will never forget because of the vast variety of "herps" that can be found in the fall or spring as they journey into or out of the swamp. My favorite memory from Snake Road is my first sighting of water moccasins or cottonmouths on a field trip! Very rare in Indiana (if they do still exist in Indiana) these cottonmouths are the most common snake seen along this site! What a treat! Let Jim and our other members share their experiences in this amazing place. Be sure to attend this program! **Remember we are meeting on Oct. -

Legal Authority Over the Use of Native Amphibians and Reptiles in the United States State of the Union
STATE OF THE UNION: Legal Authority Over the Use of Native Amphibians and Reptiles in the United States STATE OF THE UNION: Legal Authority Over the Use of Native Amphibians and Reptiles in the United States Coordinating Editors Priya Nanjappa1 and Paulette M. Conrad2 Editorial Assistants Randi Logsdon3, Cara Allen3, Brian Todd4, and Betsy Bolster3 1Association of Fish & Wildlife Agencies Washington, DC 2Nevada Department of Wildlife Las Vegas, NV 3California Department of Fish and Game Sacramento, CA 4University of California-Davis Davis, CA ACKNOWLEDGEMENTS WE THANK THE FOLLOWING PARTNERS FOR FUNDING AND IN-KIND CONTRIBUTIONS RELATED TO THE DEVELOPMENT, EDITING, AND PRODUCTION OF THIS DOCUMENT: US Fish & Wildlife Service Competitive State Wildlife Grant Program funding for “Amphibian & Reptile Conservation Need” proposal, with its five primary partner states: l Missouri Department of Conservation l Nevada Department of Wildlife l California Department of Fish and Game l Georgia Department of Natural Resources l Michigan Department of Natural Resources Association of Fish & Wildlife Agencies Missouri Conservation Heritage Foundation Arizona Game and Fish Department US Fish & Wildlife Service, International Affairs, International Wildlife Trade Program DJ Case & Associates Special thanks to Victor Young for his skill and assistance in graphic design for this document. 2009 Amphibian & Reptile Regulatory Summit Planning Team: Polly Conrad (Nevada Department of Wildlife), Gene Elms (Arizona Game and Fish Department), Mike Harris (Georgia Department of Natural Resources), Captain Linda Harrison (Florida Fish and Wildlife Conservation Commission), Priya Nanjappa (Association of Fish & Wildlife Agencies), Matt Wagner (Texas Parks and Wildlife Department), and Captain John West (since retired, Florida Fish and Wildlife Conservation Commission) Nanjappa, P. -

Life History Account for Gilbert's Skink
California Wildlife Habitat Relationships System California Department of Fish and Wildlife California Interagency Wildlife Task Group GILBERT'S SKINK Plestiodon gilberti Family: SCINCIDAE Order: SQUAMATA Class: REPTILIA R037 Written by: S. Morey Reviewed by: T. Papenfuss Edited by: R. Duke Updated by: CWHR Program Staff, March 2000 DISTRIBUTION, ABUNDANCE, AND SEASONALITY A common but seldom observed lizard, the Gilbert's skink is found in the northern San Joaquin Valley, in the Sierra Nevada Foothills from Yuba Co. southward, and along the inner flanks of the Coast Ranges from San Francisco Bay to the Mexican border. It is also found in the mountains of southern California, and at scattered mountain localities in the eastern deserts from Mono Co. to San Bernardino Co. Its elevational range is from sea level to at least 2220 m (7300 ft) (Stebbins 1985). Found in a wide variety of habitats, this lizard is most common in early successional stages or open areas within habitats in which it occurs. Heavy brush and densely forested areas are generally avoided. SPECIFIC HABITAT REQUIREMENTS Feeding: This skink, like the western skink, forages through leaf litter and dense vegetation, occasionally digging through loose soil. Stebbins (1954) suggested that its food habits are similar to those of the western skink, which takes a large percentage of ground dwelling insects. Cover: Cover for these secretive lizards is provided by rotting logs, surface litter, and large flat stones. Gilbert's skinks are good burrowers and often construct their own shelters by burrowing under surface objects. Reproduction: Females construct nest chambers in loose moist soil several cm deep under surface objects, especially flat rocks. -

Ecography ECOG-03593 Tarr, S., Meiri, S., Hicks, J
Ecography ECOG-03593 Tarr, S., Meiri, S., Hicks, J. J. and Algar, A. C. 2018. A biogeographic reversal in sexual size dimorphism along a continental temperature gradient. – Ecography doi: 10.1111/ecog.03593 Supplementary material SUPPLEMENTARY MATERIAL A biogeographic reversal in sexual size dimorphism along a continental temperature gradient Appendix 1: Supplementary Tables and Figures Table A1. Placement of species missing from phylogeny. Species Comment Reference Most closely related to oaxaca and Campbell, J.A., et al. 2016. A new species of Abronia mixteca, most similar to mixteca Abronia cuetzpali (Squamata: Anguidae) from the Sierra Madre del Sur of according to Campbell et al. so add Oaxaca, Mexico. Journal of Herpetology 50: 149-156. as sister to mixteca Anolis alocomyos Both formerly part of tropidolepis, Köhler, G., et al. 2014. Two new species of the Norops & Anolis make a random clade with pachypus complex (Squamata, Dactyloidae) from Costa leditzigorum tropidolepis Rica. Mesoamerican Herpetology 1: 254–280. Part of a clade with microtus and Poe S, Ryan M.J. 2017. Description of two new species Anolis brooksi & ginaelisae so make a random clade similar to Anolis insignis (Squamata: Iguanidae) and Anolis kathydayae with these & brooksi & kathydayae, resurrection of Anolis (Diaphoranolis) brooksi. Amphibian based on Poe & Ryan. & Reptile Conservation 11: 1–16. Part of a clade with aquaticus and Köhler, J.J., et al. 2015. Anolis marsupialis Taylor 1956, a Anolis woodi so make a random clade with valid species from southern Pacific Costa Rica (Reptilia, marsupialis these Squamata, Dactyloidae). Zootaxa 3915111–122 Köhler, G., et al. 2016. Taxonomic revision of the Norops Anolis mccraniei, Formerly part of tropidonotus, so tropidonotus complex (Squamata, Dactyloidae), with the Anolis spilorhipis, split tropidonotus into a random resurrection of N. -

Western Riverside County Regional Conservation Authority (RCA) Annual Report to the Wildlife Agencies
Western Riverside County Multiple Species Habitat Conservation Plan (MSHCP) Biological Monitoring Program Stream Survey Report 2009 23 April 2010 Stream Survey Report 2009 TABLE OF CONTENTS INTRODUCTION.........................................................................................................................................1 GOALS AND OBJECTIVES ...................................................................................................................2 METHODS ....................................................................................................................................................2 PROTOCOL DEVELOPMENT ................................................................................................................2 PERSONNEL AND TRAINING ...............................................................................................................3 STUDY SITE SELECTION.....................................................................................................................3 SURVEY METHODS ............................................................................................................................4 COVERED SPECIES....................................................................................................................5 NON-COVERED SPECIES ............................................................................................................5 RESULTS.......................................................................................................................................................5 -

Phase I Cultural and Paleontological Assessment: Magnolia Tank Farm Project City of Huntington Beach, Orange County, California
PHASE I CULTURAL AND PALEONTOLOGICAL ASSESSMENT: MAGNOLIA TANK FARM PROJECT CITY OF HUNTINGTON BEACH, ORANGE COUNTY, CALIFORNIA Prepared on Behalf of: SLF-HB Magnolia, LLC 2 Park Plaza Irvine, CA 92614 Principal Investigators/Authors: Tria Marie Belcourt, M.A., Registered Professional Archaeologist Jennifer Kelly, M.Sc., Geology, Professional Paleontologist Sonia Sifuentes, M.Sc., Registered Professional Archaeologist Material Culture Consulting Project Number: SRI-17-01 Type of Study: Cultural and paleontological resources assessment Cultural Resources within Area of Potential Impact: None Paleontological Formations: Younger Quaternary Alluvium USGS Quadrangle: Newport Beach APN(s): 114-150-36, 114-481-32 Survey Area: 29 acres Date of Survey: August 8, 2017 Key Words: Paleontology, Archaeology, CEQA, Phase I Survey, Negative Survey MANAGEMENT SUMMARY SLF-HB Magnolia, LLC proposes to convert a currently vacant and graded lot into a mixed-use community that provides visitor serving commercial uses, new residential neighborhoods, opportunities for coastal access and passive recreation and incorporates measures to protect adjacent natural resources, called the Magnolia Tank Farm Project (Project). The Project is located in the City of Huntington Beach, Orange County, California The Project includes construction of up to 250 for-sale residential units and visitor-and-resident-serving commercial uses facilities. Material Culture Consulting, Inc. (Material Culture) was retained by SLF-HB Magnolia, LLC (SLF-HB) to conduct the Phase I cultural and paleontological resource investigation of the Project Area. These assessments were conducted in accordance with the California Environmental Quality Act (CEQA), and included cultural and paleontological records searches, a search of the Sacred Lands File by the Native American Heritage Commission (NAHC), outreach efforts with ten Native American tribal representatives, background research, and a pedestrian field survey, all of which resulted in negative findings. -

East Tennessee State University Digital Commons@ East
East Tennessee State University Digital Commons @ East Tennessee State University Electronic Theses and Dissertations Student Works 5-2011 On The rC anial Osteology of Eremiascincus and Its Use For Identification. William B. Gelnaw East Tennessee State University Follow this and additional works at: https://dc.etsu.edu/etd Part of the Paleontology Commons Recommended Citation Gelnaw, William B., "On The rC anial Osteology of Eremiascincus and Its Use For Identification." (2011). Electronic Theses and Dissertations. Paper 1294. https://dc.etsu.edu/etd/1294 This Thesis - Open Access is brought to you for free and open access by the Student Works at Digital Commons @ East Tennessee State University. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Digital Commons @ East Tennessee State University. For more information, please contact [email protected]. On the Cranial Osteology of Eremiascincus, and Its Use for Identification ________________________________________ A thesis presented to the faculty of the department of Biological Sciences East Tennessee State University In partial fulfillment of the requirements for the degree Master of Sciences in Biology _______________________________________ by William B. Gelnaw May 2011 _______________________________________ James Mead, Chair Blaine Schubert Stephen Wallace Keywords: Squamata, Morphometrics, Lizards, Skull ABSTRACT On the Cranial Osteology of Eremiascincus, and Its Use for Identification by William B. Gelnaw A persistent problem